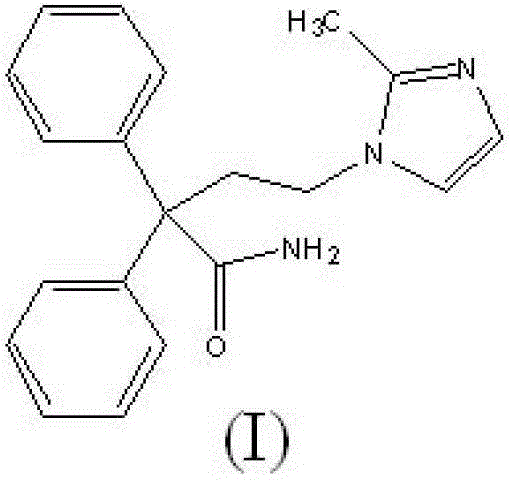
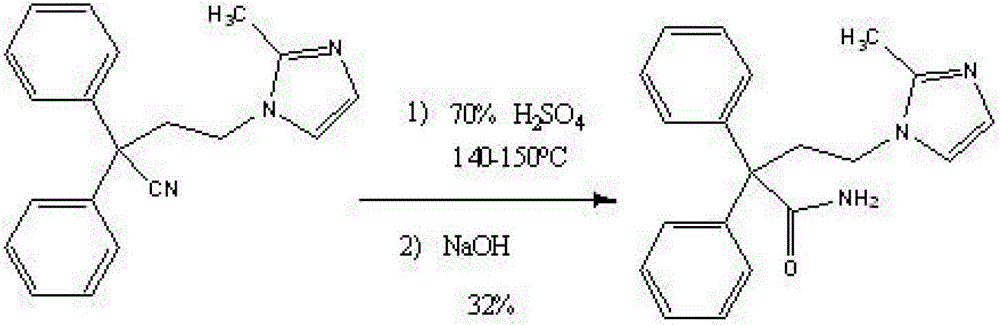
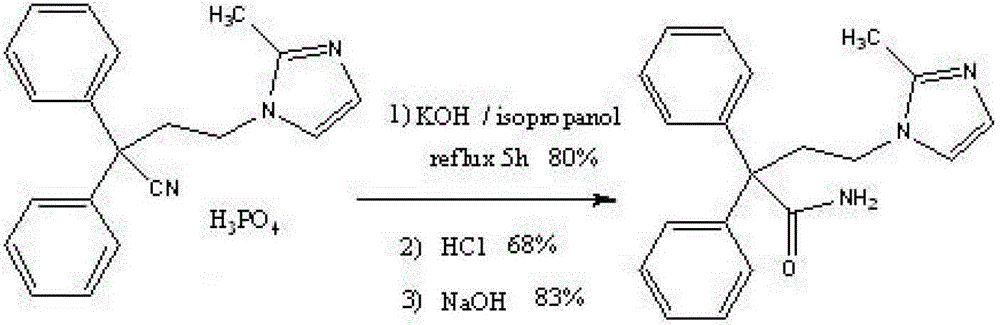
Improved method for preparing imidafenacin
A technology for compounds and substances, applied in the field of preparation of midanacin, can solve problems such as troublesome product purification, and achieve the effect of avoiding the use of a large amount of acid and alkali
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] In a 50ml three-neck flask, add methanol 10ml, 4-(2-methylimidazol-1-yl)-2,2-diphenylbutyronitrile phosphate 2.0g (5mmol), DMSO1.17g (15mmol), at room temperature Add 1.7g of 30% hydrogen peroxide (the actual amount of hydrogen peroxide is 15mmol), under stirring, slowly add 5mol / L sodium hydroxide solution (about 5ml) dropwise, control the reaction temperature not to exceed 30°C, and raise the temperature to 50°C after adding, Stir for 3 hours, TLC shows that the reaction is complete (developing solvent: ethyl acetate), add 30ml of water, stir for 1h at an internal temperature below 15°C, filter, wash with water, and recrystallize the crude product with 90% ethanol (1g crude product is used 6ml solvent), to obtain about 1.21g of white powdery solid, yield 76%.
[0029] 1H NMR (CDCl3): δ2.228(3H, s), δ2.711(2H, t), δ3.787(2H, t), δ5.344(1H, brs), δ5.636(1H, brs), δ6.724(1H,s), δ6.850(1H,s), δ7.314-7.408(10H,m)
Embodiment 2
[0031] In a 50ml three-necked flask, add 10ml of ethanol, 2.0g (5mmol) of 4-(2-methylimidazol-1-yl)-2,2-diphenylbutyronitrile phosphate, 1.56g (20mmol) of DMSO, at room temperature Add 1.13g of 30% hydrogen peroxide (the actual amount of hydrogen peroxide is 10mmol), and slowly add 30% sodium carbonate solution (about 5ml) dropwise under stirring. hours, TLC shows that the reaction is complete (developing solvent: ethyl acetate), add 30ml of water, stir for 1h at an internal temperature below 15°C, filter, rinse with water, and recrystallize the obtained crude product with 90% ethanol (1g crude product with 6ml solvent ), about 1.15 g of white powdery solid was obtained, with a yield of 72%.
Embodiment 3
[0033] In a 50ml three-neck flask, add methanol 10ml, 4-(2-methylimidazol-1-yl)-2,2-diphenylbutyronitrile phosphate 2.0g (5mmol), DMSO1.17g (15mmol), at room temperature Add 2.26g of 30% hydrogen peroxide (the actual amount of hydrogen peroxide is 20mmol), under stirring, slowly add 5mol / L potassium hydroxide solution (about 5ml) dropwise, control the reaction temperature not to exceed 30°C, and raise the temperature to 40°C after adding, Stir for 2 hours, TLC shows that the reaction is complete (developing solvent: ethyl acetate), add 30ml of water, stir for 1h at an internal temperature below 15°C, filter, rinse with water, and recrystallize the obtained crude product with 90% ethanol (1g crude product is used 6ml solvent), to obtain about 1.19g of white powdery solid, yield 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com