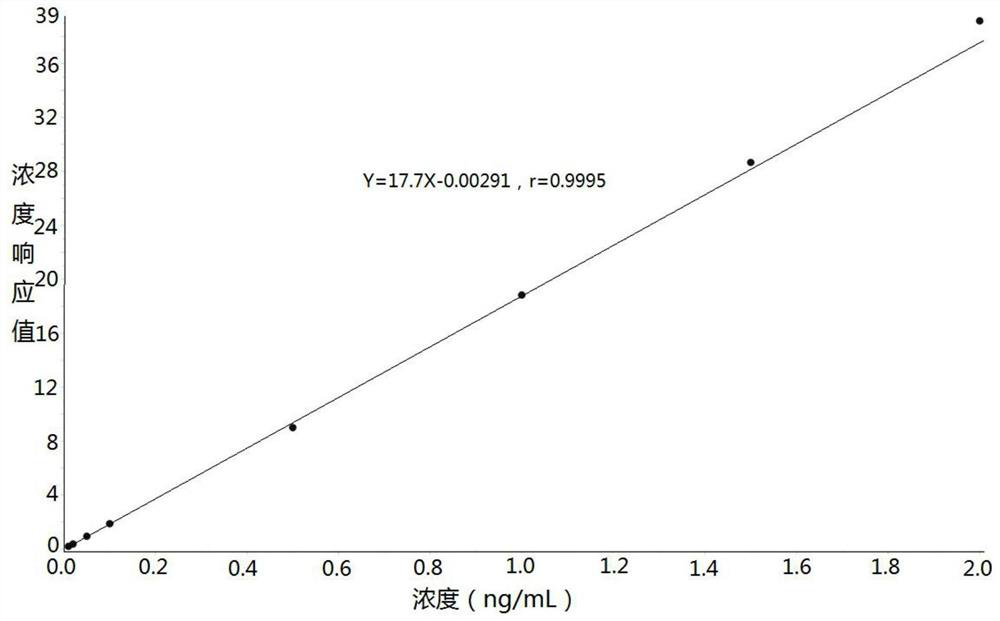
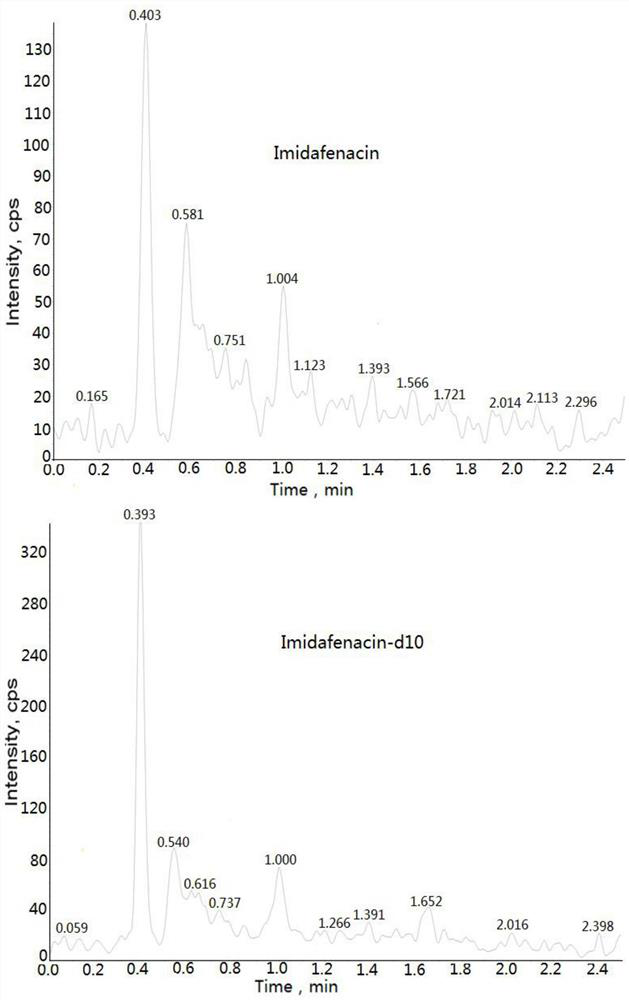
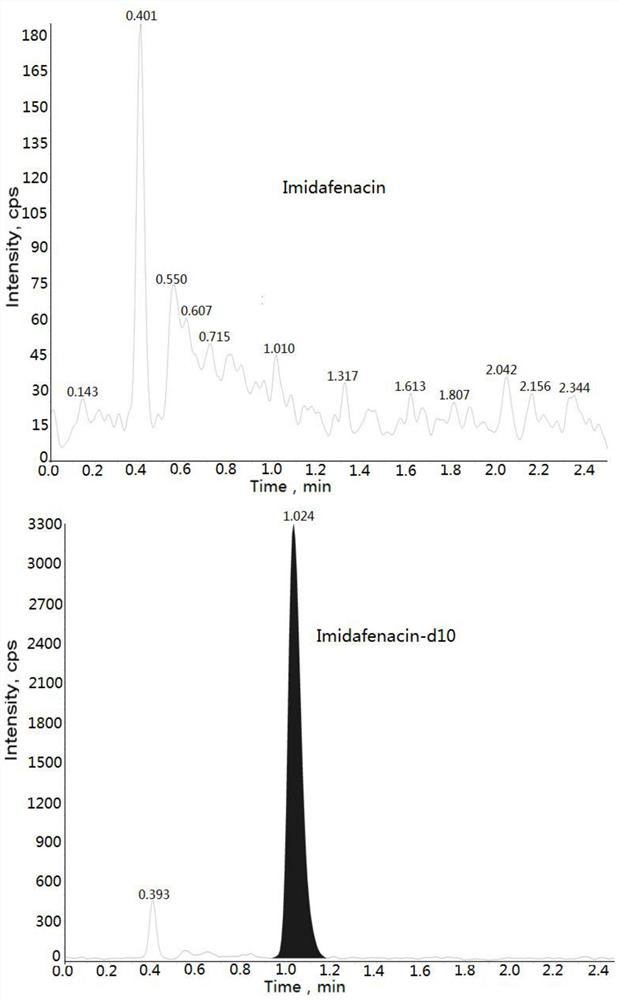
Method for determining concentration of imidafenacin in blood plasma by liquid chromatography-mass spectrometry
A midanacin, LC/MS technology, applied in the field of medicine, can solve the problems of speed, precision, sensitivity, selectivity, etc. to be improved, and achieve the effect of high sensitivity, strong specificity, and stable baseline
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0032] Example: Human K 2 Determination of Midanacin Concentration in EDTA Plasma
[0033] 1. Experimental materials and analytical equipment Midanacin (analyte): TLC Pharmaceutical Standards or the same or higher standard product Midanacin-d10 (internal standard): TLC Pharmaceutical Standards or the same or higher standard The reagents used in the product are shown in Table 1 below:
[0034] Table 1 Reagent Details
[0035] Reagent name level manufacturer Acetonitrile (ACN) HPLC J. T. Baker Ammonium acetate (CH 3 COONH 4 )
HPLC J. T. Baker Formic acid (FA) ACS Adamas Methanol (MeOH) HPLC J. T. Baker
[0036] Note: Reagents of the same grade or higher can also be used
[0037] The analytical equipment used is shown in Table 2 below:
[0038] Table 2 Details of equipment used
[0039]
[0040]
[0041] The same LC / MS / MS system can also be used.
[0042] 2. Liquid conditions
[0043] 1. Liquid chromatograph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com