Method for determining concentration of lacosamide in blood plasma by liquid chromatography-mass spectrometry
A technology of lacosamide and LC/MS, applied in the field of medicine, can solve the problems of speed, precision, sensitivity, selectivity, etc. to be improved, and achieve the effects of simple pretreatment method, rapid method and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
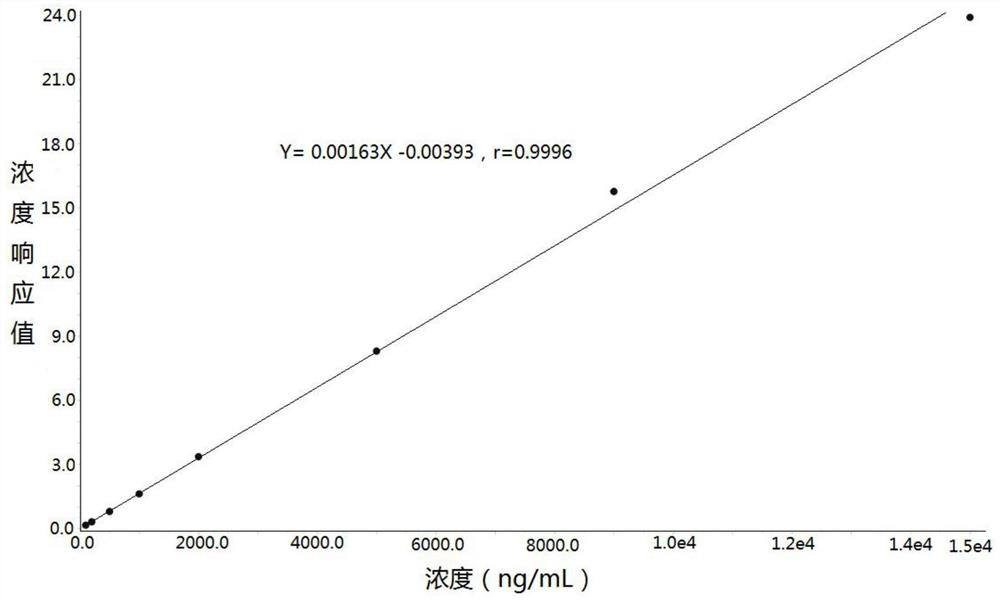
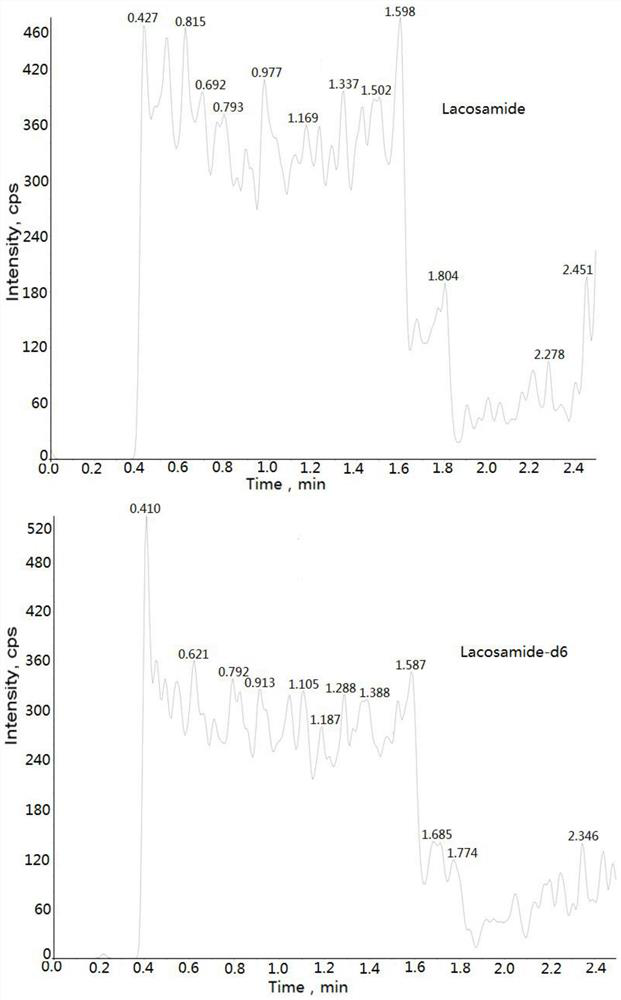
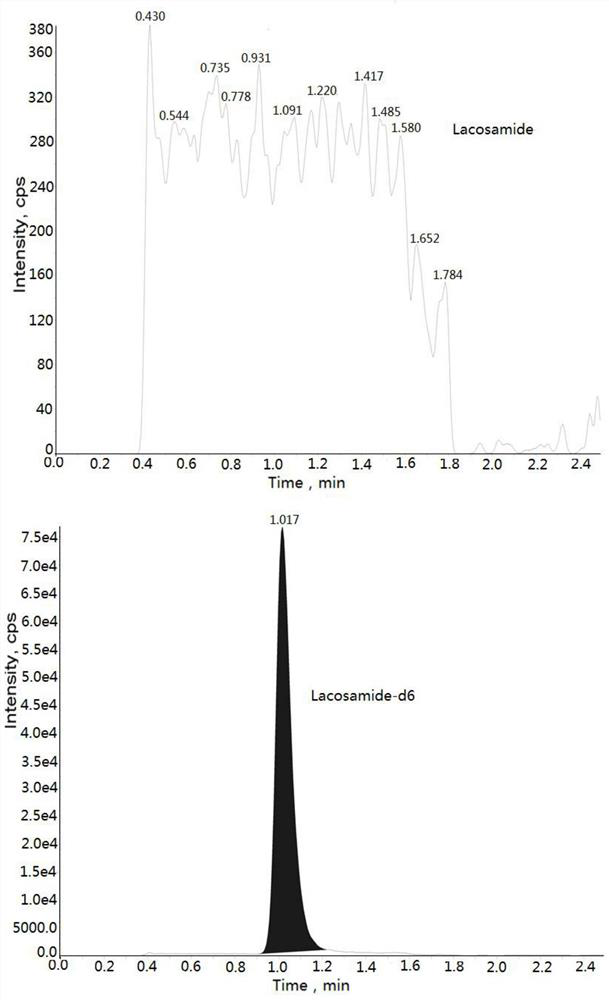
[0032] Example: Human K 2 Determination of Lacosamide Concentration in EDTA Plasma
[0033] 1. Experimental materials and analytical equipment Lacosamide (analyte): TLC Pharmaceutical Standards or the same or higher standard Lacosamide-d6 (internal standard): TLC Pharmaceutical Standards or the same or higher standard The reagents used in the product are shown in Table 1 below:
[0034] Table 1 Reagent Details
[0035] Reagent name level manufacturer Acetonitrile (ACN) HPLC J. T. Baker Ammonium acetate (CH 3 COONH 4 )
HPLC J. T. Baker Methanol (MeOH) HPLC J. T. Baker
[0036] Note: Reagents of the same grade or higher can also be used
[0037] The analytical equipment used is shown in Table 2 below:
[0038] Table 2 Details of equipment used
[0039] components model manufacturer Binary pump (binary pump) AC Pump AB SCIEX Degasser Degasser AB SCIEX Column oven AC Column oven AB SCI...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com