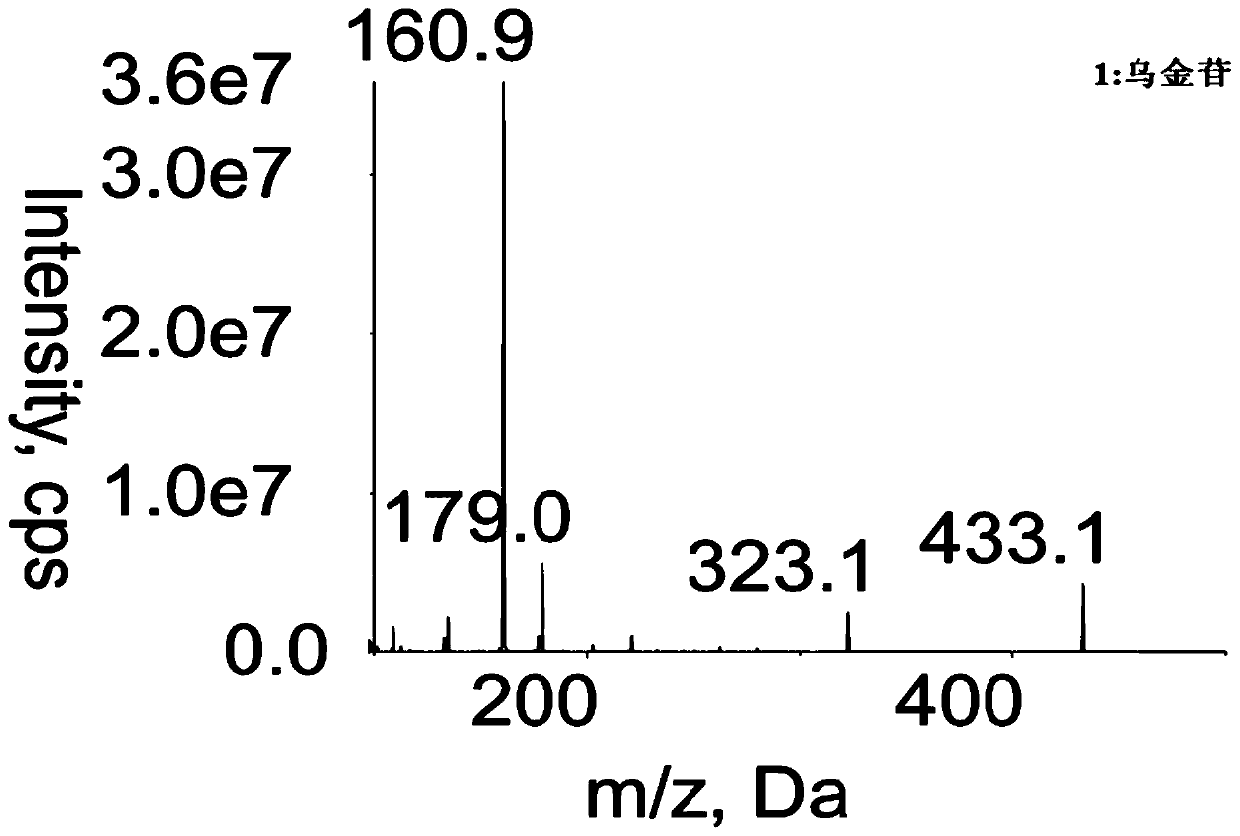
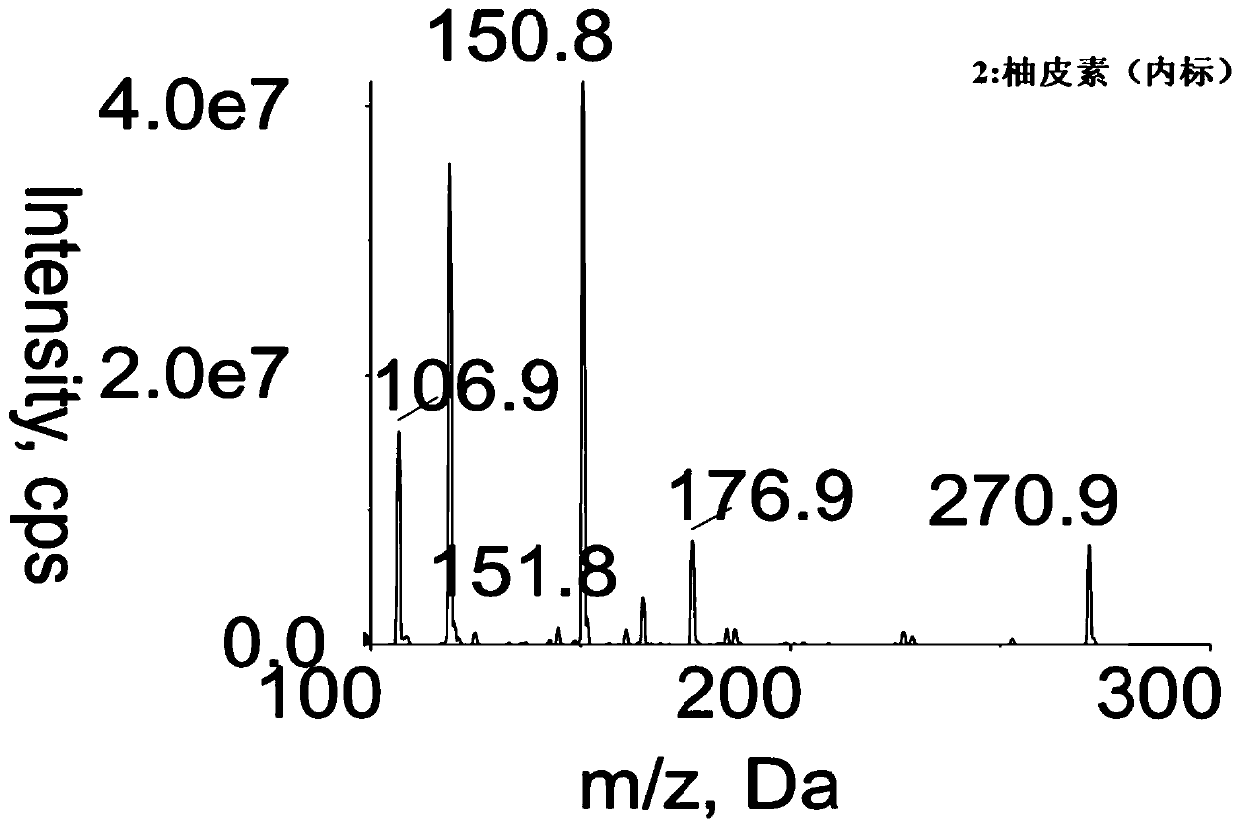
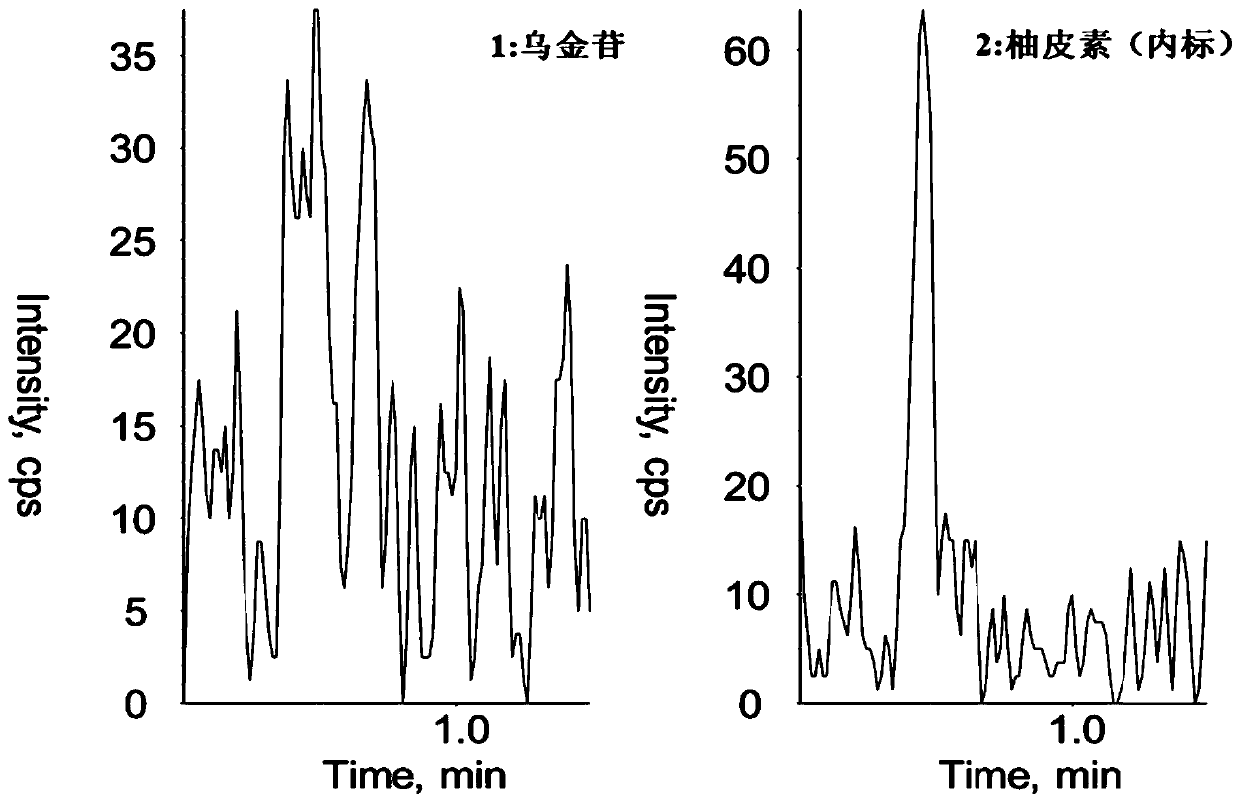
Method for determining concentration of robustaside B in blood plasma
A technology of oujinin and blood plasma, which is applied in the field of determination of oujinin concentration in plasma, and achieves the effects of strong specificity, high sensitivity, and simple pretreatment method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0029] Determination of Urinin Concentration in Rat Plasma
[0030] 1. Experimental materials and instruments
[0031] Both oujinin (MUST-18081702) and naringenin (MUST-19032406) were purchased from Chengdu Mansite Biotechnology Co., Ltd. Test water: ultrapure water; methanol: chromatographically pure (Merck Company).
[0032] API 4000LC-MS / MS triple quadrupole mass spectrometer, equipped with electrospray ionization source (ESI), chromatography workstation: Analyst 1.6; Agilent 1200 high performance liquid chromatography; AE240 electronic balance (Shanghai Mettler-Toledo Co., Ltd. company); MICRO-17R refrigerated centrifuge (Thermo, USA); WH-2 micro-vortex mixer (Shanghai Huxi Analytical Instrument Factory); Drict-Q5 ultrapure water machine (Millipore, France).
[0033] 2. Liquid conditions
[0034] 1. Liquid chromatography conditions
[0035] Chromatographic column: Waters XTerra MS C18 column (dp 3.5μm, 50mm ID×2.1mm); mobile phase: acetonitrile-2mmol L -1 Ammonium acet...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com