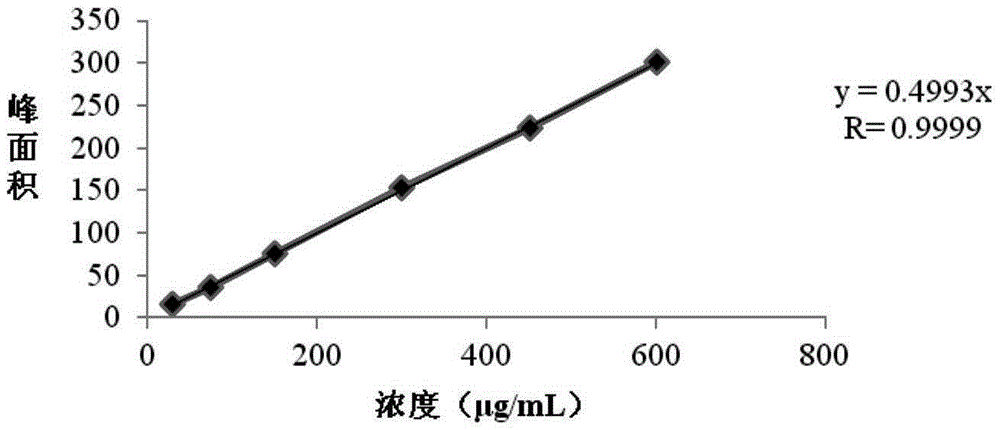
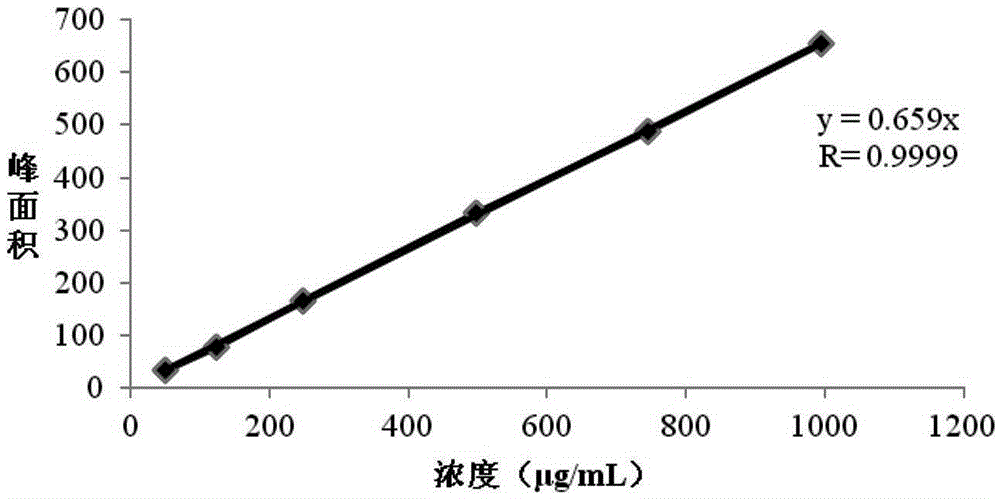
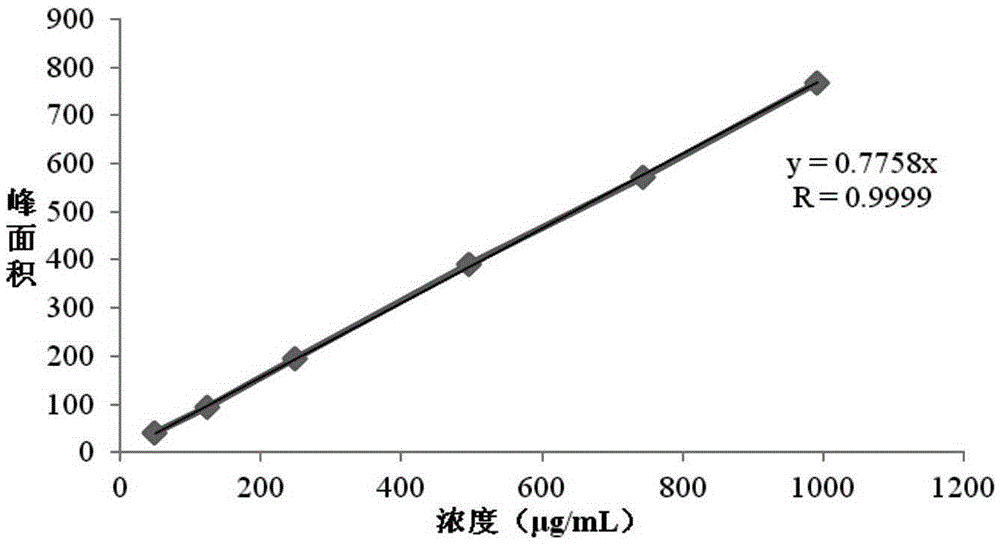
Detection method of residual solvents in (N-(3-chloro-4-(3-fluorobenzyloxy)phenyl-6-(3-(4-methyl-4-oxo-1-nitrogen-4-phosphorus hetero yclohexane-1-yl)propyl-1-alkynyl)quinazoline-4-amine, bis 4-methyl benzenesulfonate bulk drug
A kind of technology of toluenesulfonic acid generation and detection method, which is applied in the field of analytical chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Investigate the system applicability of the method provided by the present invention:
[0052] Continuous injection of 6 standard solutions. By repeatedly measuring the peak area of each solvent in the standard solution 6 times, and calculating the relative standard deviation RSD of the 6 peak areas, the RSD value is examined to judge the applicability of the system. The specific method is:
[0053] 1. Instrument
[0054] Gas chromatograph model: Agilent6890A
[0055] Headspace sampler model: Agilent7694E
[0056] 2. Chromatographic conditions: as shown in Table 1, headspace sampling conditions as shown in Table 2:
[0057] Table 1 Chromatographic conditions
[0058]
[0059] Table 2 headspace sampler parameters
[0060] Equilibrium temperature (℃)
[0061] 3 Preparation of solution
[0062] Blank solution: Accurately pipette 10ml of dimethyl sulfoxide (DMSO) into a 20ml headspace vial.
[0063] Preparation of the control stock solution: Accurately...
Embodiment 2
[0075] The specificity and selectivity investigation is mainly to investigate the identification and selectivity of peaks, and its requirements: only the blank solution and the positioning solution of all solvents are used to determine all potential impurities, and the separation between the main peak and adjacent chromatographic peaks must not less than 1.5.
[0076] Instrument, chromatographic condition, solution are identical with embodiment 1, and experimental result is as shown in table 4~5:
[0077] Table 4 Residual Solvent Positioning Solution Determination Results
[0078] remaining solvent
[0079] Table 5 mixed standard solution measurement results
[0080] remaining solvent
[0081] Conclusion: The test shows that the samples measured by this method have good results, and the resolution is greater than 1.5.
Embodiment 3
[0083] Prepare a mixed reference solution of methanol 63.09 μg / ml, ethanol 100.49 μg / ml, isopropanol 102.39 μg / ml and DMF solution 19.05 μg / ml. Dilute the above solutions to appropriate concentrations, respectively, take 10mL and inject them into 20mL headspace vials. For these known residual solvents, the limit of quantitation was determined by calculation of the signal-to-noise ratio. Dilute the stock solution of the known residual solvent to a certain concentration, compare the measured response signal with the response signal in the blank, and calculate the true and reliable minimum concentration that can be detected. Instrument, chromatographic condition, solution are identical with embodiment 1, and detection result is as shown in table 6:
[0084] Table 6: Quantitation limit test results
[0085] test solution
[0086] The results show that the method provided by the invention has a lower detection limit, indicating higher sensitivity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com