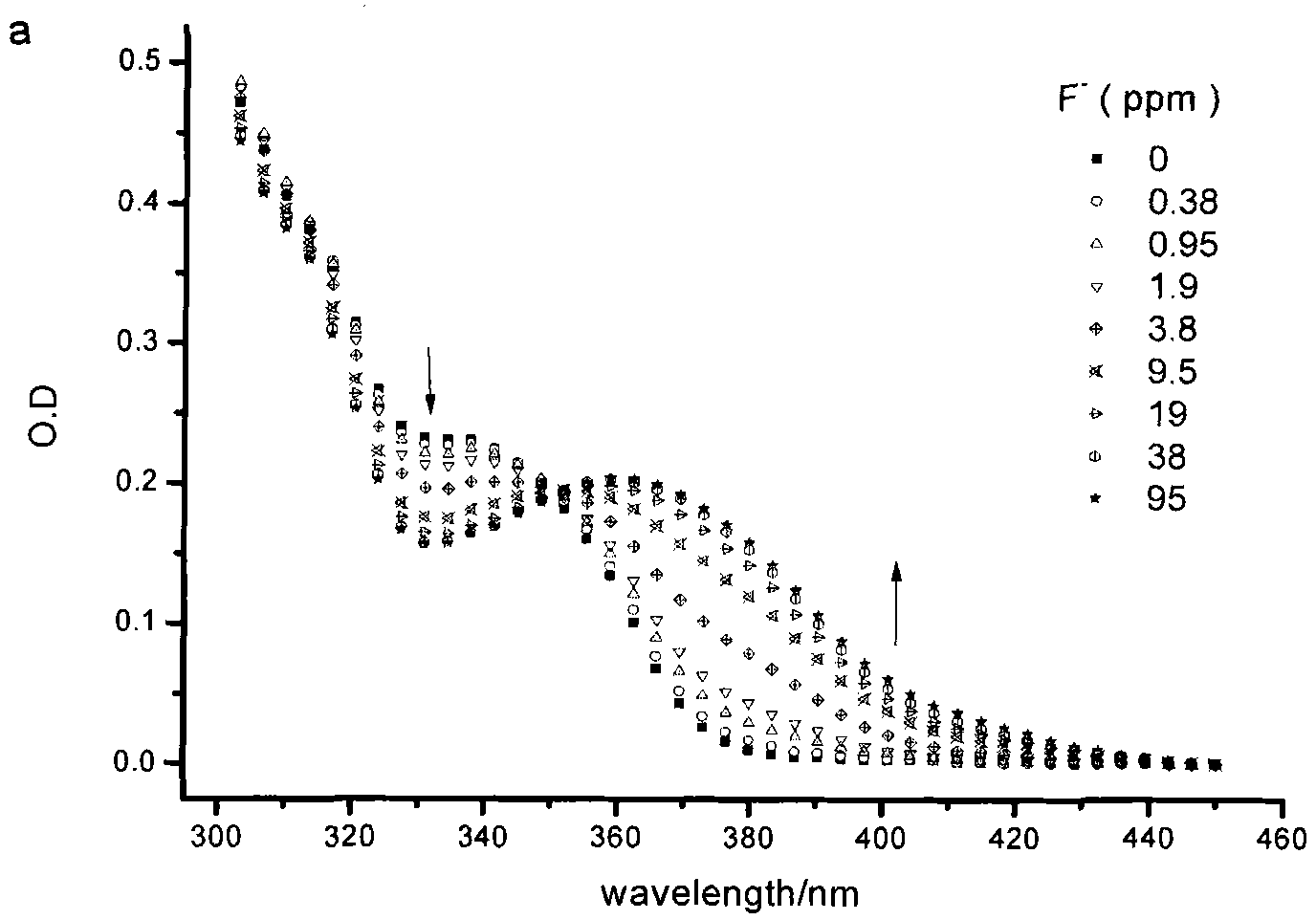
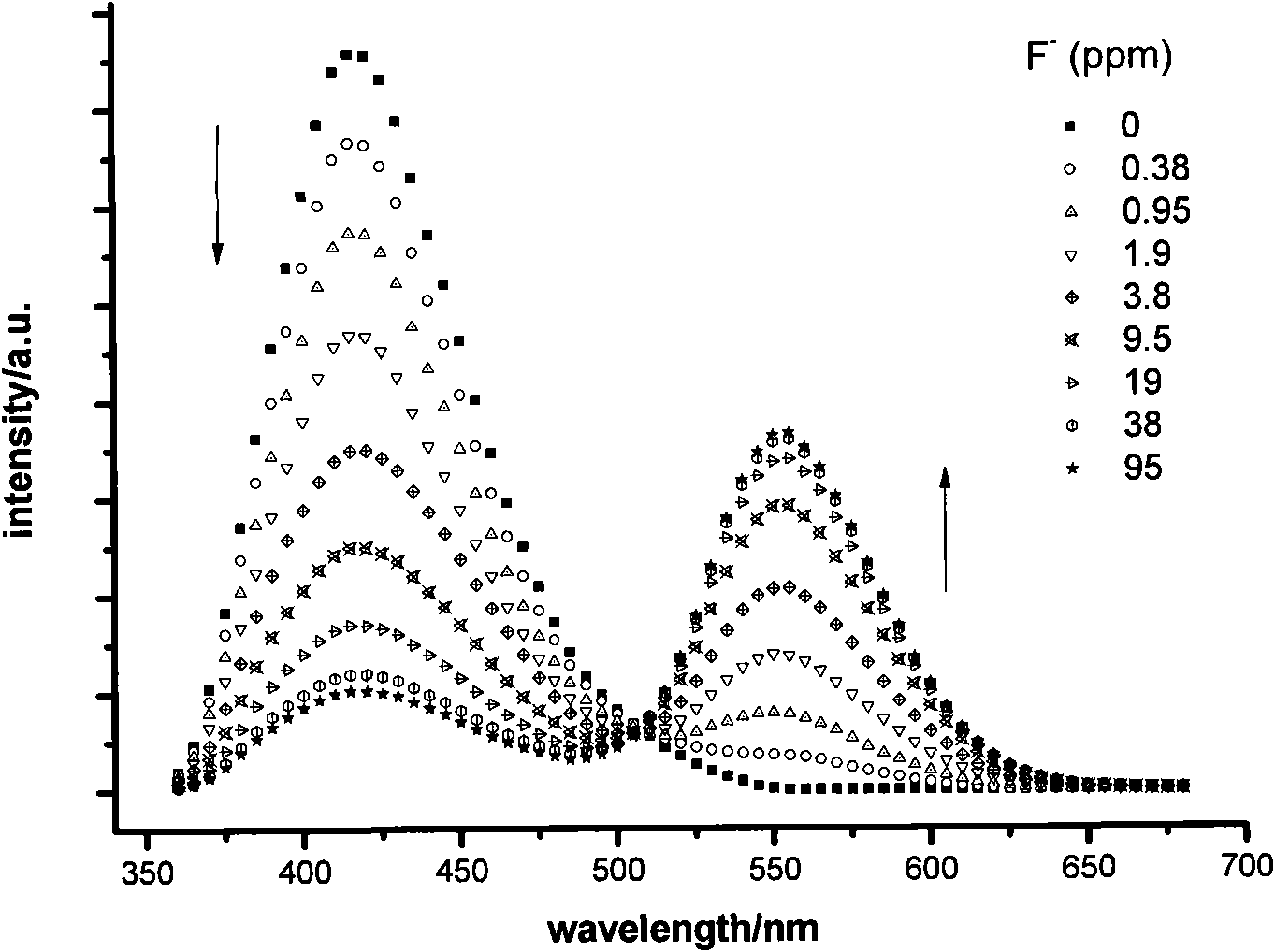
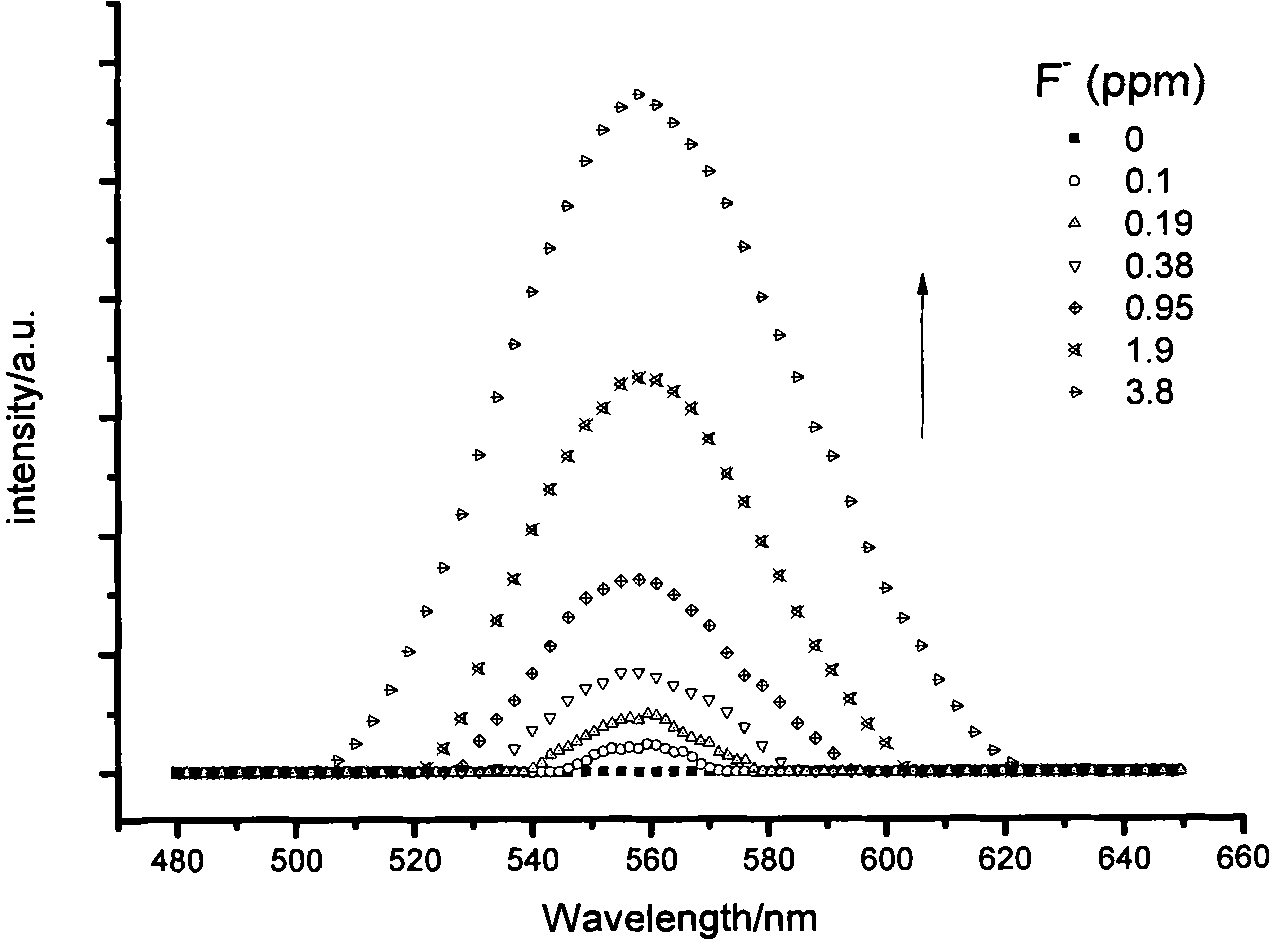
Fluorescent probe for identifying fluorine ions and preparation method and use thereof
A technology of fluoride ions and compounds, applied in the field of fluorescent probes, can solve problems such as quantitative measurement problems, difficulty in applying water systems, and interference of fluorescent probes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Embodiment 1, preparation compound IIC, its molecular structural formula is:
[0096]
[0097] where X=S,
[0098] Its reaction scheme is as follows:
[0099]
[0100]Add 602 mg of benzoic acid and 972 mg of 1,1'-carbonyldiimidazole to 30 ml of toluene, under nitrogen protection, reflux and stir for 6 hours, after the reaction is cooled, add 2-(5-amino-2-hydroxyphenyl ) After the benzothiazole, the reaction was continued to reflux for 6 hours. The reaction solution was filtered, respectively, with CH 2 Cl 2 and CH 3 OH washing and drying to obtain the product.
[0101] Compound IIC: 1 H-NMR (400MHz, d 6 -DMSO) δ 11.39(s, 1H), 10.32(s, 1H), 8.67(s, 1H), 8.16(d, J=7.92Hz, 1H), 8.07(d, J=8.20Hz, 1H), 8.00(d, J=7.80Hz, 2H), 7.86(d, J=3.24Hz, 1H), 7.62-7.53(m, 4H), 7.46(t, J=7.52Hz, 1H), 7.09(d, J =8.84Hz, 1H).ESI-MS (m / z): [M+H] + 347.1. Elemental analysis (C 20 h 14 N 2 o 2 S): C, 69.35%; H, 4.07%; N, 8.09%, measured: C, 69.20%; H, 4.20%; N, 8.11%.
...
Embodiment 2
[0106] Embodiment 2, preparation compound IC, its molecular structural formula is:
[0107]
[0108] where X=S, R 4 tert-butyl
[0109] The specific reflection route is as follows:
[0110] Dissolve 350mg of compound IIC in 20ml of THF, add excess metal sodium, stir at room temperature for 4 hours, remove excess sodium, add twice the amount of tert-butyl-2-phenyl chloride to the remaining suspension Silane, stirred and refluxed overnight, and the volatile components were removed under reduced pressure, and the residue was separated through a silica chromatography plate. The eluent was: THF-cyclohexane (1:7, v / v) to obtain the final product. The above operation All carried out under anhydrous and oxygen-free conditions. 1 H-NMR (400MHz, CDCl 3 )δ8.32(s, 1H), 8.13(d, J=8.11Hz, 1H), 7.98(d, J=8.05Hz, 1H), 7.77-7.83(m, 7H), 7.60(d, J=8.52 Hz, 1H), 7.37-7.53(tqq, J=7.49, 6.73, 7.06Hz, 11H), 6.59(d, J=9.02Hz, 1H), 1.18(s, 9H).MALDI-TOF MS(m / z ): [M+H] + 585.3. Elemental ...
Embodiment 3
[0115] Embodiment 3, preparation of compound IC nanoparticles:
[0116] Configure the THF solution of the compound IC synthesized in Example 2, and the concentration is: 2.0×10 -3 M, at the same time prepare a CTAB aqueous solution with a concentration of 1mM, take 10ml for later use; quickly inject 100 μl of THF solution of Compound IC into the stirred 10ml CTAB aqueous solution to prepare the nanoparticles of Compound IC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com