Method for detecting impurity F and impurity G of omeprazole sodium
A technology of omeprazole sodium and a detection method, applied in the field of pharmaceutical preparations, can solve the problems of multiple impurities, inaccurate impurities, easy changes in chemical structure and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
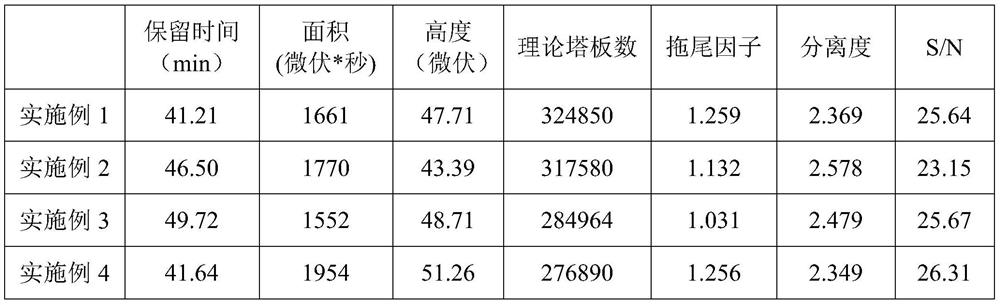
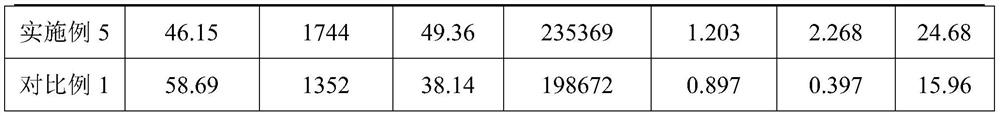
Embodiment 1
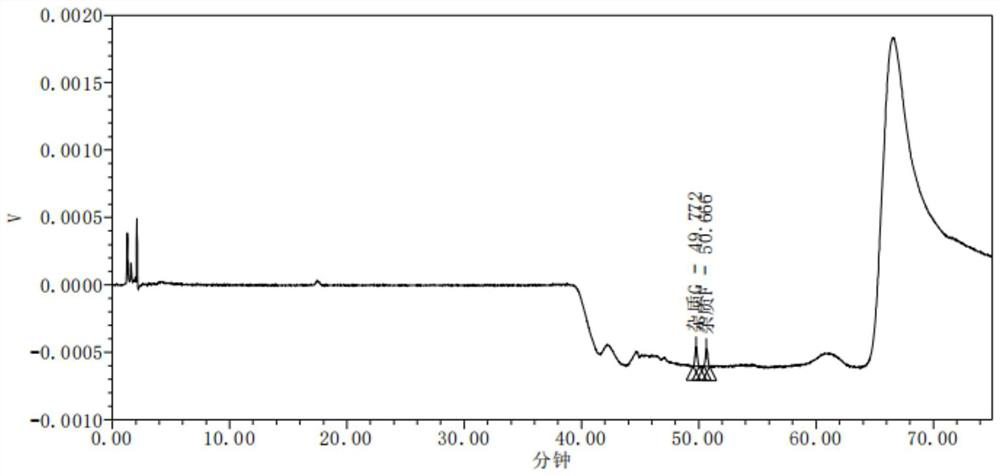
[0029] A detection method of omeprazole sodium impurity F and impurity G, comprising the steps of: adopting high performance liquid chromatography to detect omeprazole sodium impurity F and impurity G,
[0030] (1) HPLC detection conditions are as follows:
[0031] Chromatographic column: Octylsilane bonded silica gel as filler
[0032] Mobile phase A: 0.003mol / L disodium hydrogen phosphate solution-acetonitrile, adjust the pH to 7.8, the volume ratio of disodium hydrogen phosphate and acetonitrile is 73:27
[0033] Mobile Phase B: Acetonitrile
[0034] The detection wavelength is 200nm
[0035] Column temperature is 22°C
[0036] Injection volume 15μl
[0037] Flow rate 0.8ml / min
[0038] Gradient elution was carried out with mobile phase, and the elution procedure was as follows:
[0039] From 0 to 35 minutes, the volume ratio of mobile phase A to mobile phase B is 88:12;
[0040] From 35.01 to 60 minutes, the volume ratio of mobile phase A to mobile phase B is 50:50;...
Embodiment 2
[0045] A detection method of omeprazole sodium impurity F and impurity G, comprising the steps of: adopting high performance liquid chromatography to detect omeprazole sodium impurity F and impurity G,
[0046] (1) HPLC detection conditions are as follows:
[0047] Chromatographic column: Octylsilane bonded silica gel as filler
[0048] Mobile phase A: 0.012mol / L disodium hydrogen phosphate solution-acetonitrile, adjust the pH to 7.8, the volume ratio of disodium hydrogen phosphate and acetonitrile is 73:27
[0049] Mobile phase B: acetonitrile aqueous solution with a mass fraction of 0.5-1.3%
[0050] The detection wavelength is 300nm
[0051] Column temperature is 28°C
[0052] Injection volume 25μl
[0053] Flow rate 1.2ml / min
[0054] Gradient elution was carried out with mobile phase, and the elution procedure was as follows:
[0055] From 0 to 35 minutes, the volume ratio of mobile phase A to mobile phase B is 99:1;
[0056] From 35.01 to 60 minutes, the volume ra...
Embodiment 3
[0061] A detection method of omeprazole sodium impurity F and impurity G, comprising the steps of: adopting high performance liquid chromatography to detect omeprazole sodium impurity F and impurity G,
[0062] (1) HPLC detection conditions are as follows:
[0063] Chromatographic column: Octylsilane bonded silica gel as filler
[0064] Mobile phase A: 0.01mol / L disodium hydrogen phosphate solution-acetonitrile, adjust the pH to 7.8, the volume ratio of disodium hydrogen phosphate and acetonitrile is 73:27
[0065] Mobile phase B: acetonitrile aqueous solution with a mass fraction of 0.5-1.3%
[0066] The detection wavelength is 280nm
[0067] Column temperature is 25°C
[0068] Injection volume 20μl
[0069] Flow rate 1.0ml / min
[0070] Gradient elution was carried out with mobile phase, and the elution procedure was as follows:
[0071] From 0 to 35 minutes, the volume ratio of mobile phase A to mobile phase B is 90:10;
[0072] From 35.01 to 60 minutes, the volume ra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com