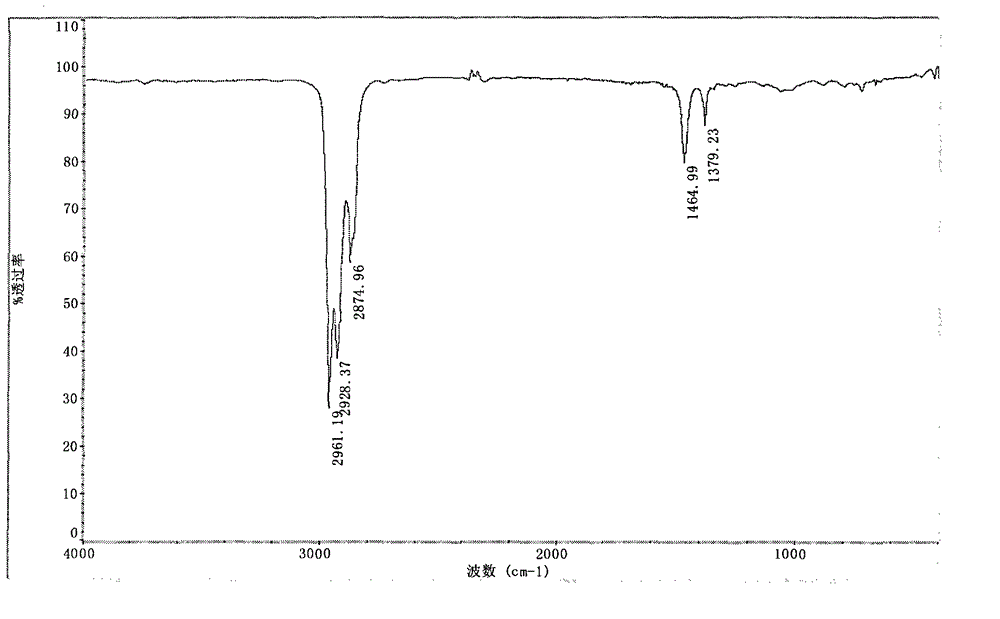
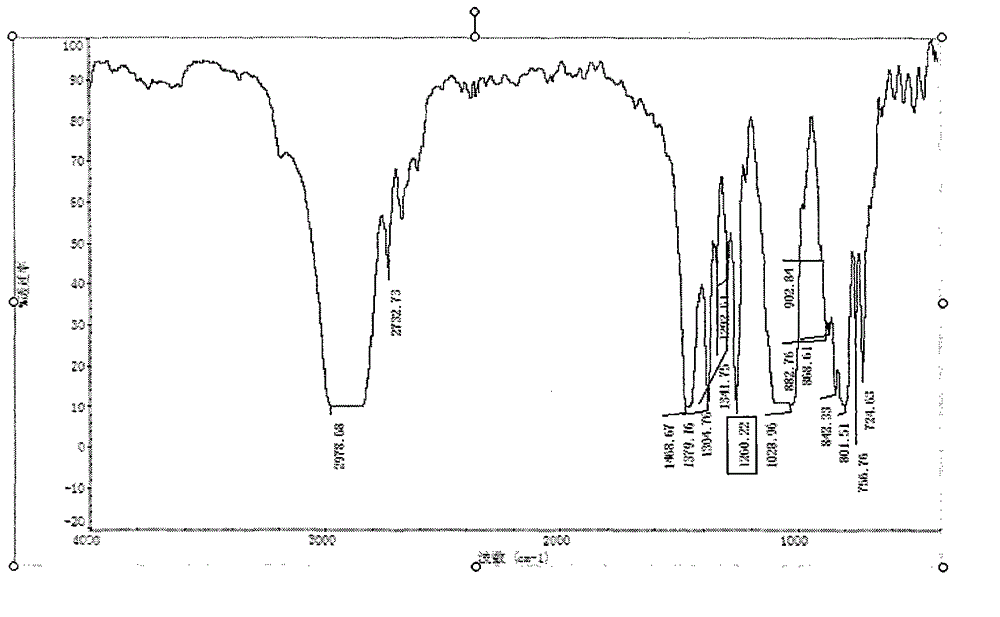
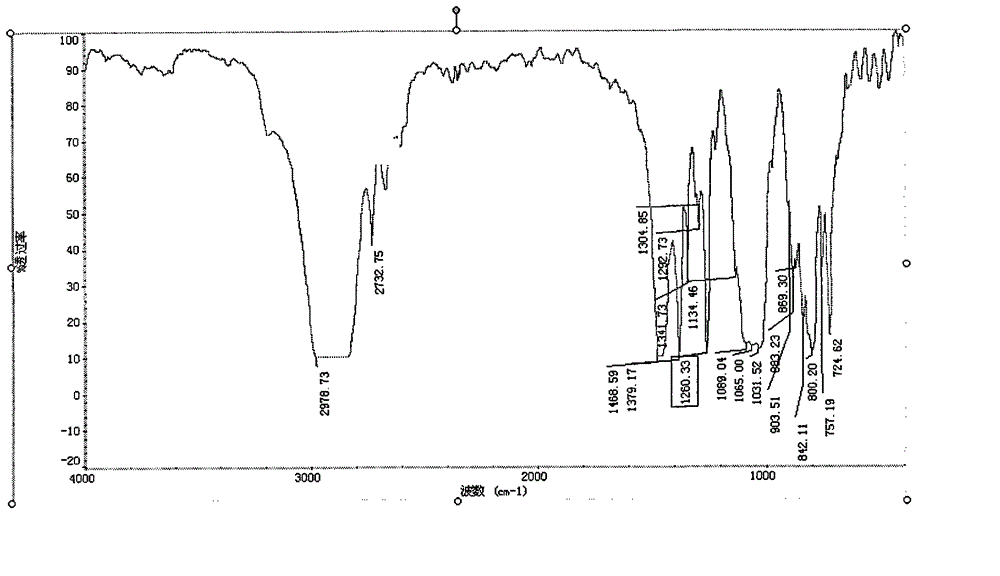
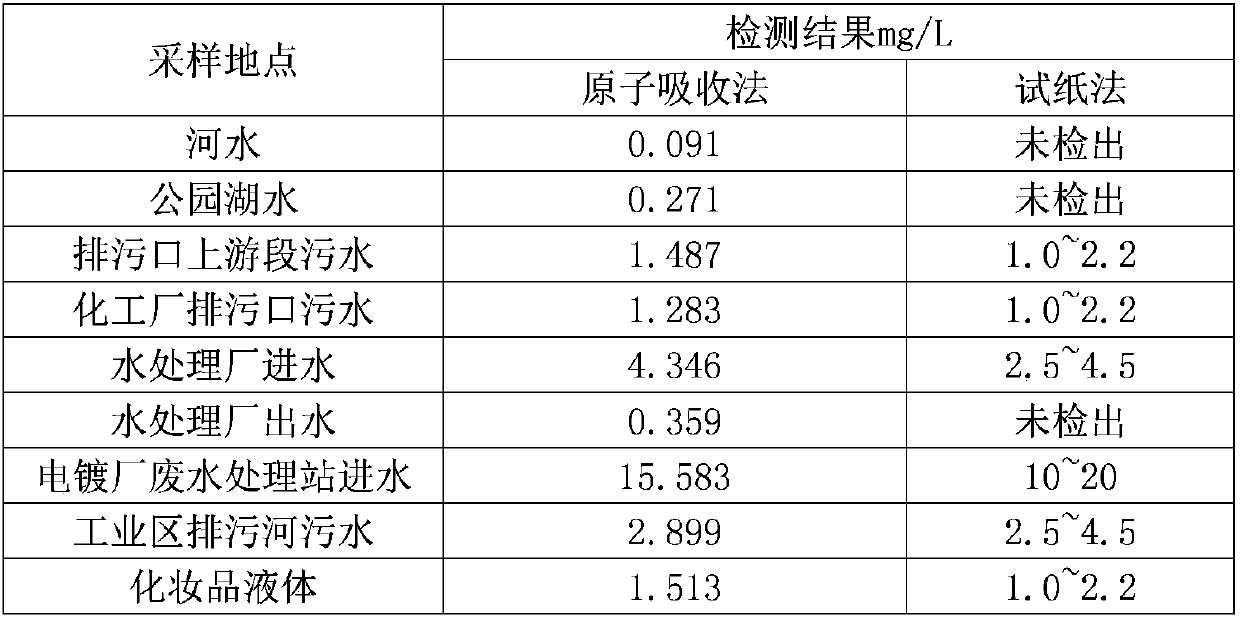
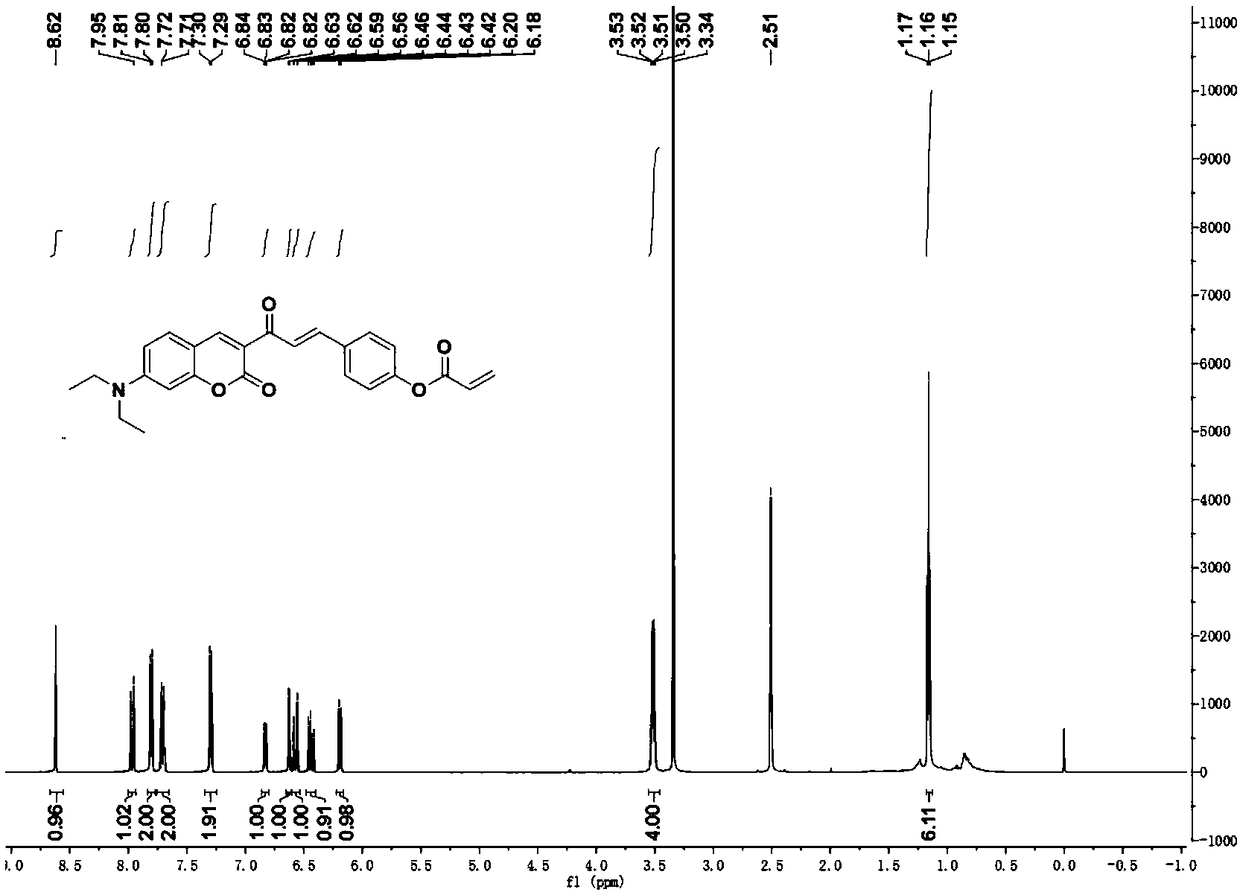
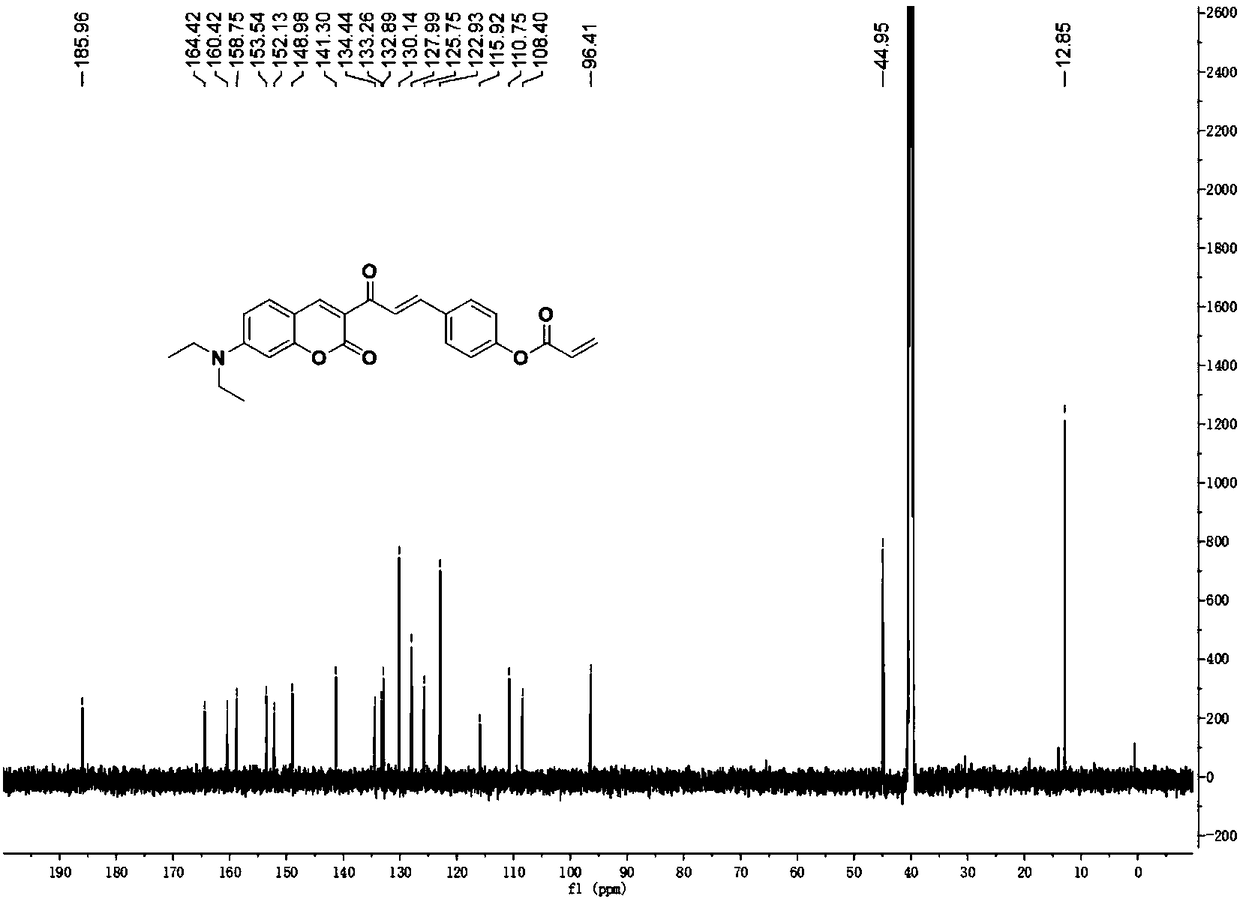
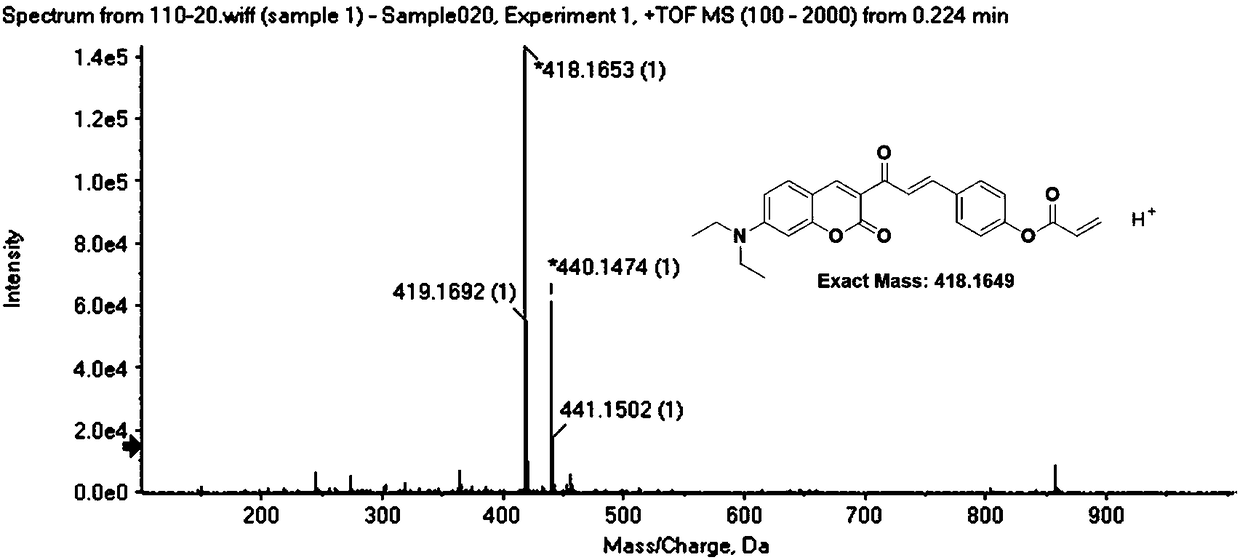
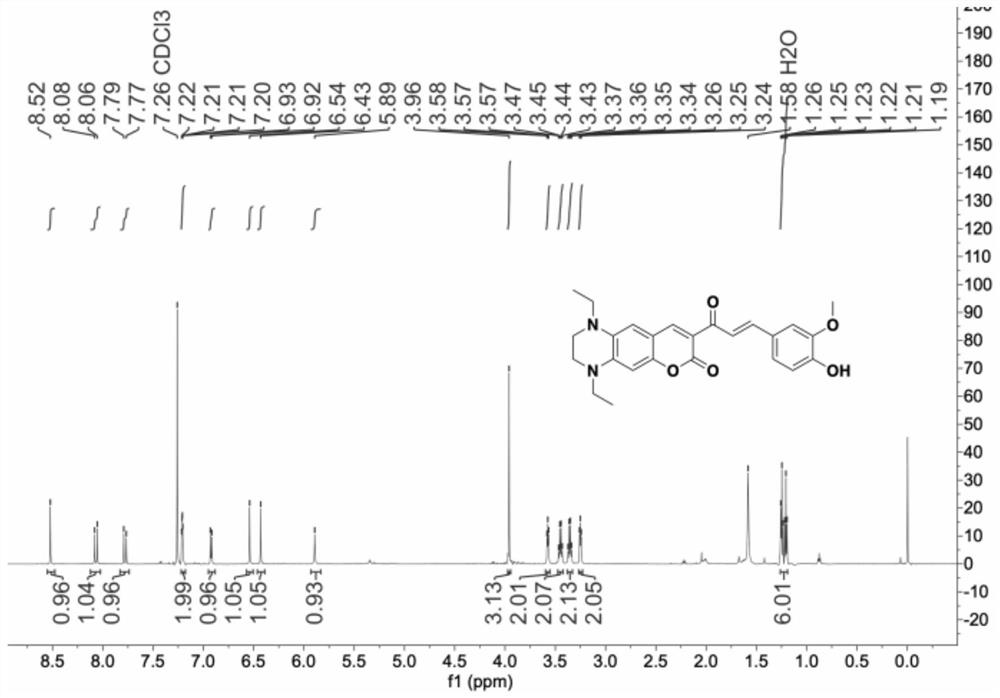
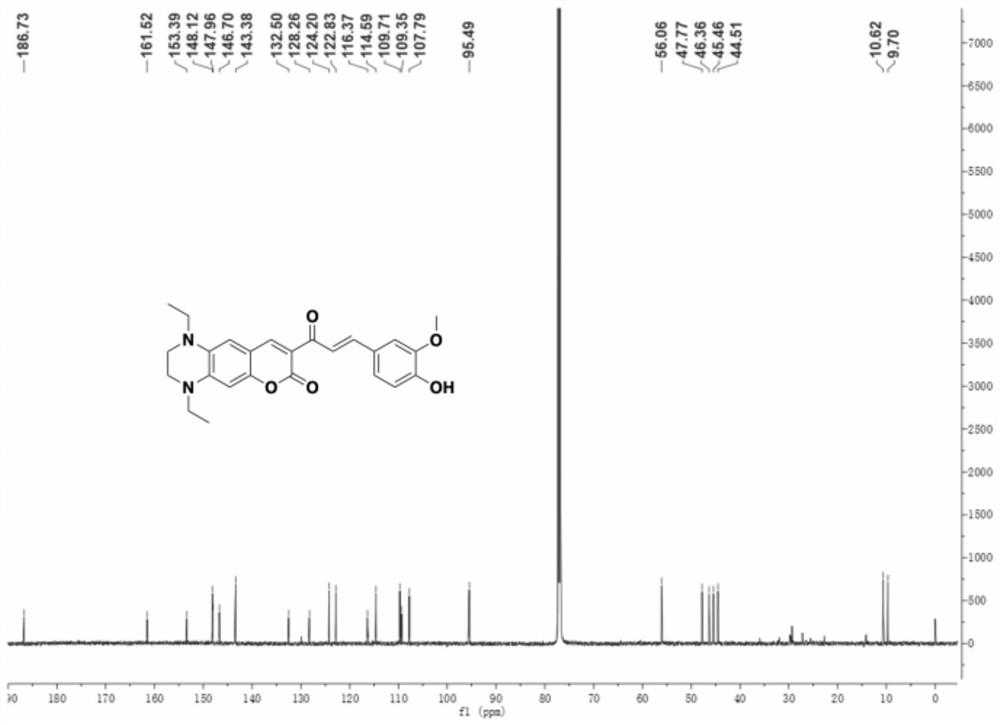
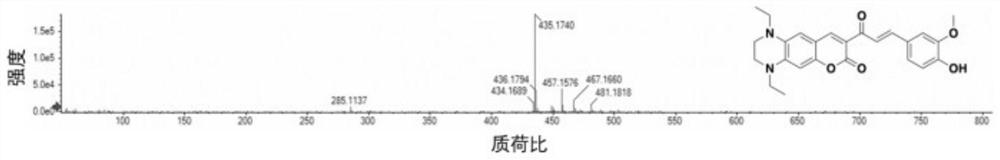
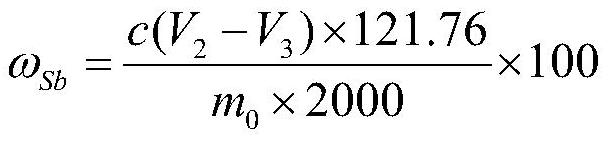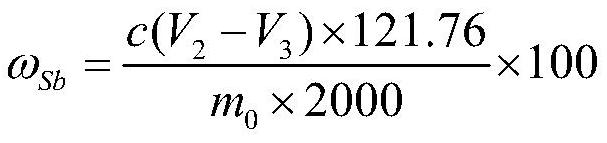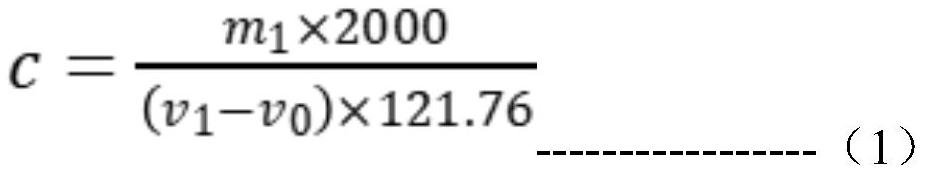Patents
Literature
33results about How to "Do not interfere with determination" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
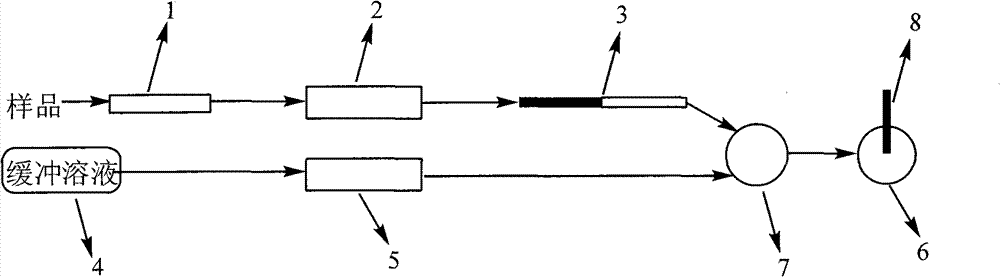
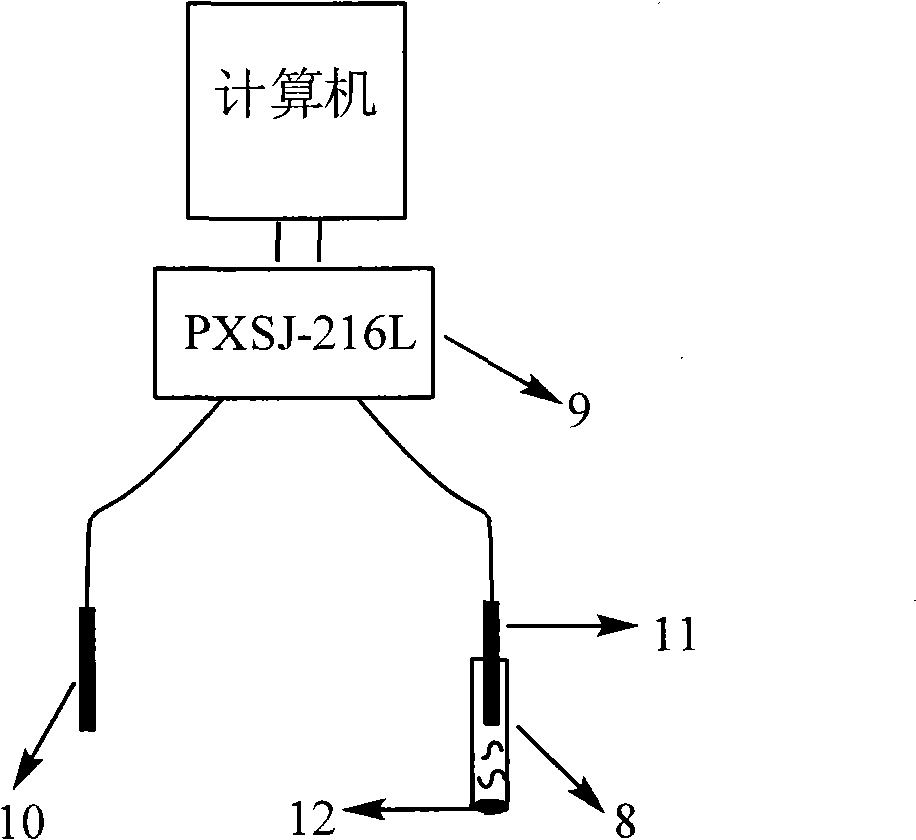
Method and device for detecting tripolycyanamide
ActiveCN101706469AReduce test costEasy to automateComponent separationMaterial analysis by electric/magnetic meansIon exchangePre treatment
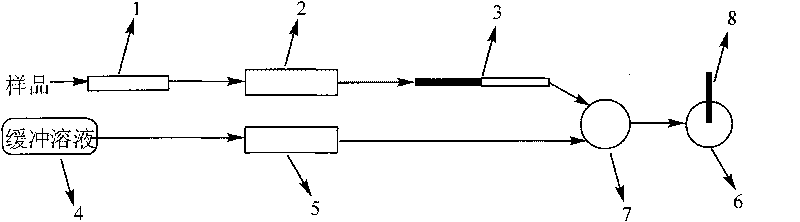
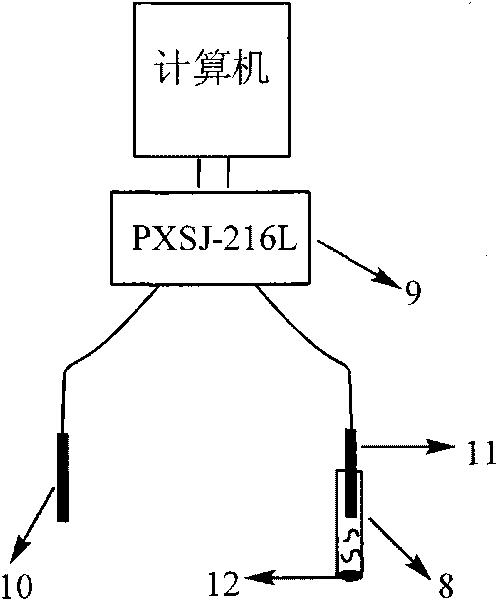
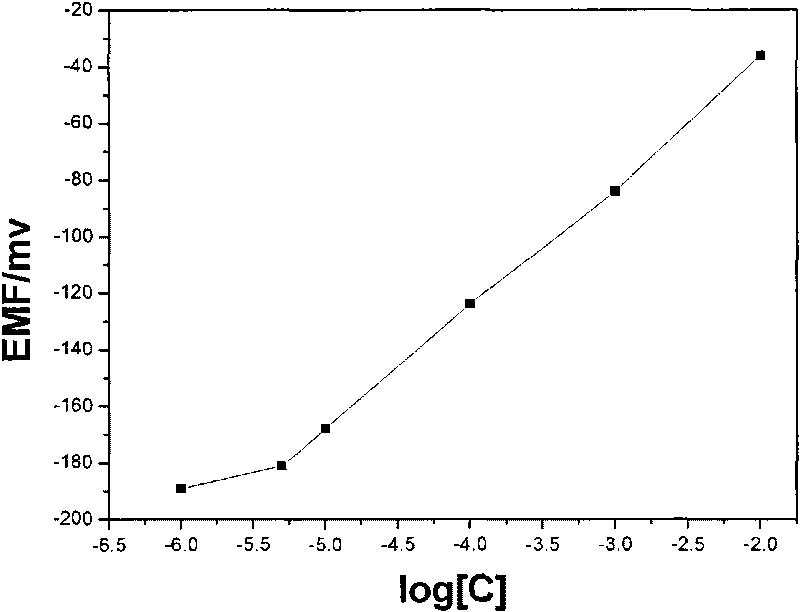
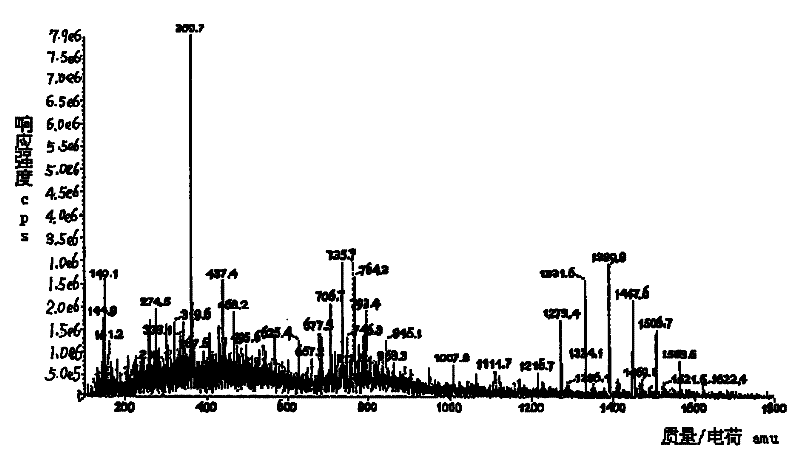
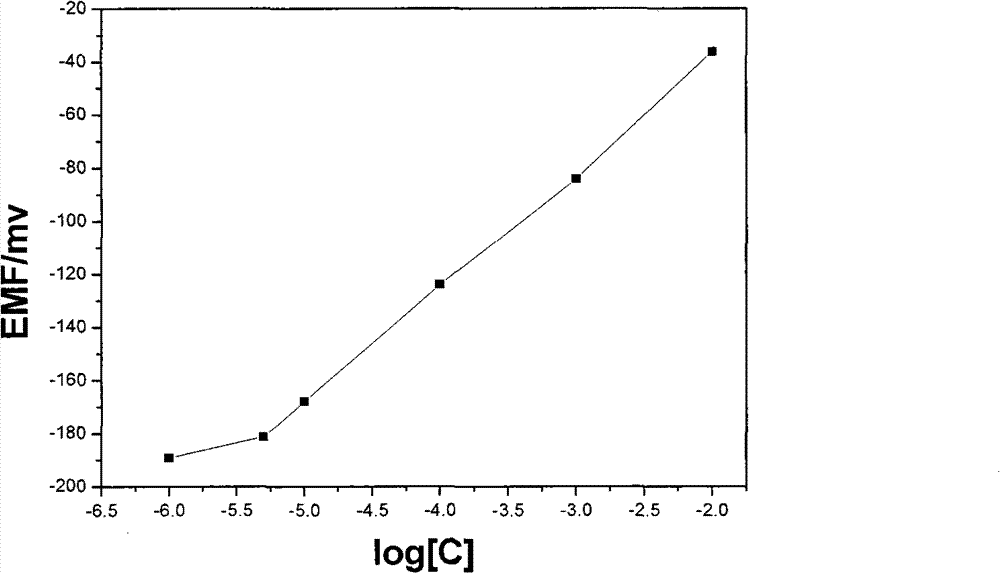
The invention relates to the detection of tripolycyanamide, in particular to a method and a device for detecting tripolycyanamide. The method comprises: preprocessing a sample to be detected to remove proteins; allowing obtained clear liquid to pass through an anionic and cationic ion exchange series column at a constant flow rate; transferring the clear liquid to be detected and buffer solution into a cell for samples to be detected; inserting an ion selective electrode modified by a molecular imprinted polymer into the cell; generating an electric potential signal; and determining the tripolycyanamide content of the sample to be detected by using an electric potential value according to a standard curve. In the device, one end of the anionic and cationic ion exchange series column, a second flow injection device and a container for the samples to be detected are connected with a three-way valve respectively; the other end of the anionic and cationic ion exchange series column is connected with an ultramicropore protein filter through a first flow injection device; and the second flow injection device is connected with a buffer solution tank; and the ion selective electrode modified by the molecular imprinted polymer is inserted into the container for the samples to be detected. The method and the device have the advantages of high flexibility, low operation cost, suitability for in-situ monitoring and the like in the detection of tripolycyanamide.
Owner:YANTAI INST OF COASTAL ZONE RES CHINESE ACAD OF SCI
Method for detecting blood concentration of multiple antiepileptic drugs simultaneously
InactiveCN1971270ASuitable for routine testingDo not interfere with determinationTesting dairy productsTesting medicinal preparationsEpoxyPretreatment method
The invention belongs to field of medical examination and relates to assay determination method of internal medicine, specially a detecting method of six antiepileptics medicines of primidone, phenylethylmalonylurea, diphenylhydantoin, carbamazepine, Larmortriazine, okazepine and the active product monohydroxy okazepine of okazepine and the active product epoxy carbamazepine of carbamazepine in human plasma. The sample to be measured is eluted equicontinuously in condition of acidic mobile phase via the pretreatment of protein precipitation, and separated by chromatographic column, and detected by ultraviolet detector. The quantity of sample in the invention is small; the pretreatment method is simple, fast and sensitive, free of expensive apparatus and agents, applicability is wide, cost is low, it fits for the detection of normal blood concentration in clinic.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
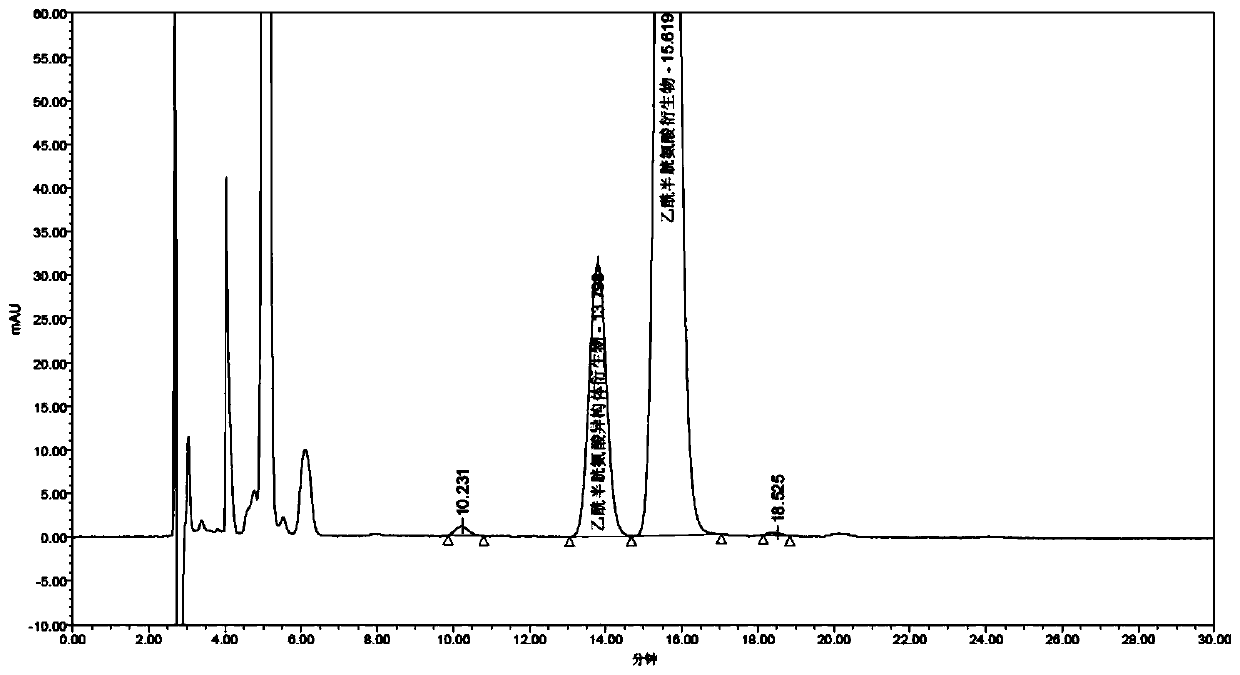
Separation and detection method of acetylcysteine enantiomer
ActiveCN109725073AIncrease mutual attractionHigh selectivityComponent separationChromatography columnChemistry
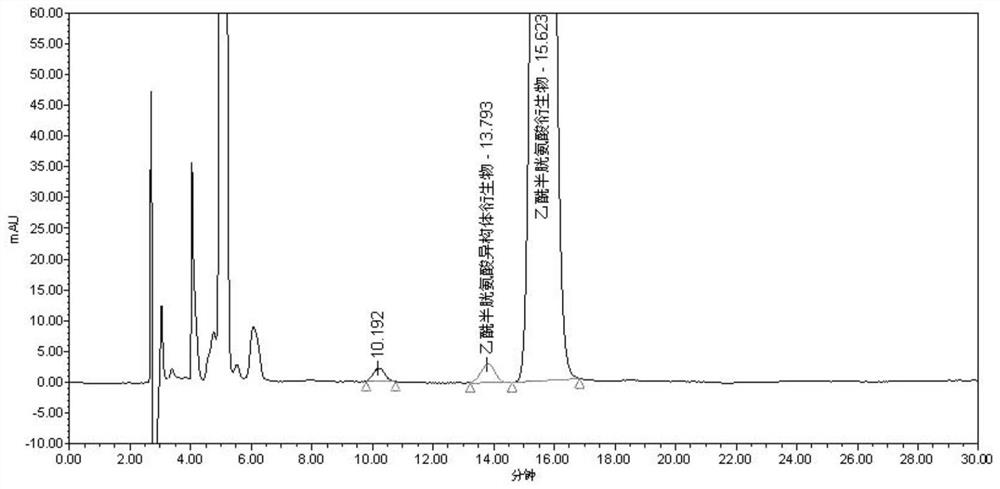
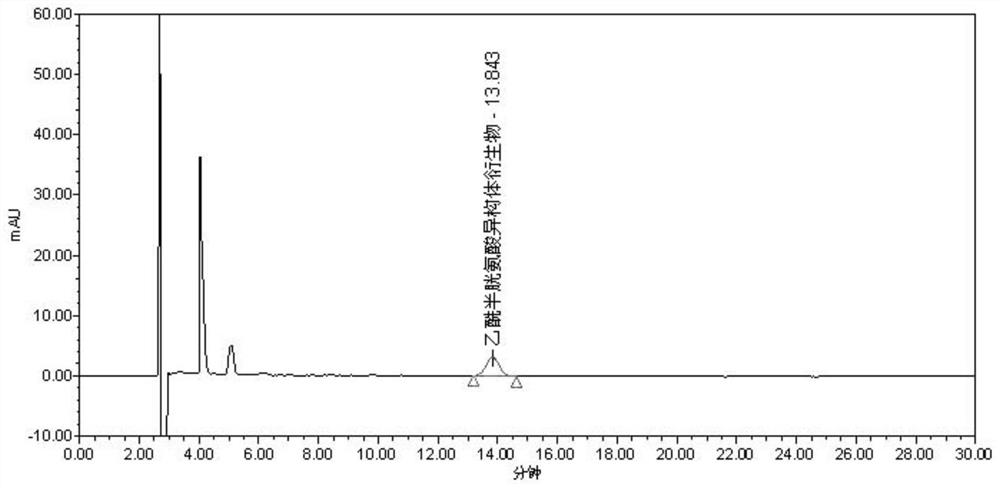
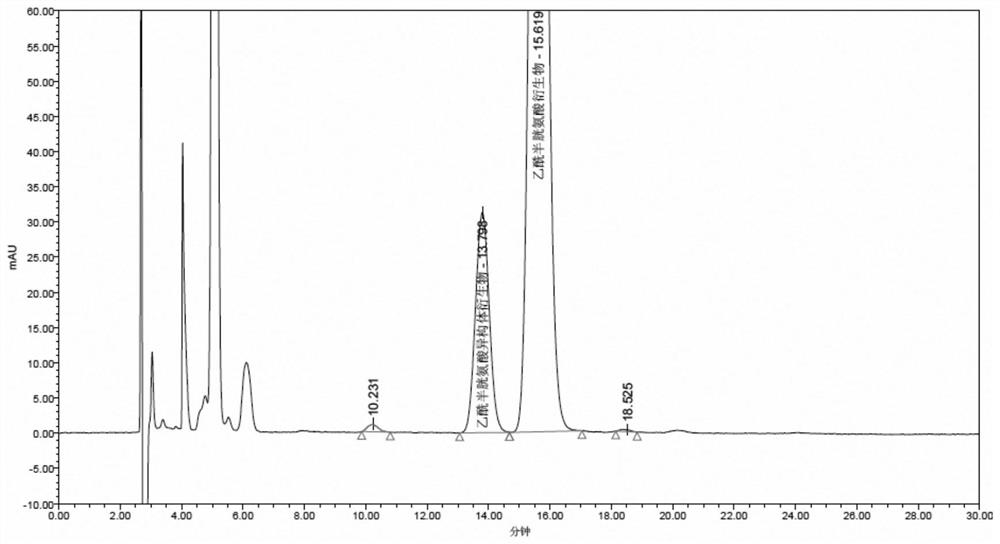
The invention discloses a separation and detection method of an acetylcysteine enantiomer. The method comprises the following steps: performing derivatization reaction in an organic solvent by using aderivatization reagent, N-acetyl-L-cysteine and an enantiomer thereof, so as to prepare a derivative product of N-acetyl-L-cysteine and isomers thereof; and performing separation and detection by using normal phase high-performance liquid chromatography, wherein the derivatization reagent is isocyanate. According to the separation and detection method of the acetylcysteine enantiomer, the derivatization reagent, N-acetyl L-cysteine and the enantiomer thereof are reacted, so that the mutual attraction effect between the stationary phase in the chromatographic column and the chiral substance tobe separated is increased according to the chiral separation mechanism by adopting precolumn derivatization normal phase high-performance liquid chromatography, and the chiral separation selectivityis effectively improved. The method is simple and convenient to operate, short in consumption time and easy to popularize and use.
Owner:HUNAN WARRANT PHARM TECH DEV CO LTD +1
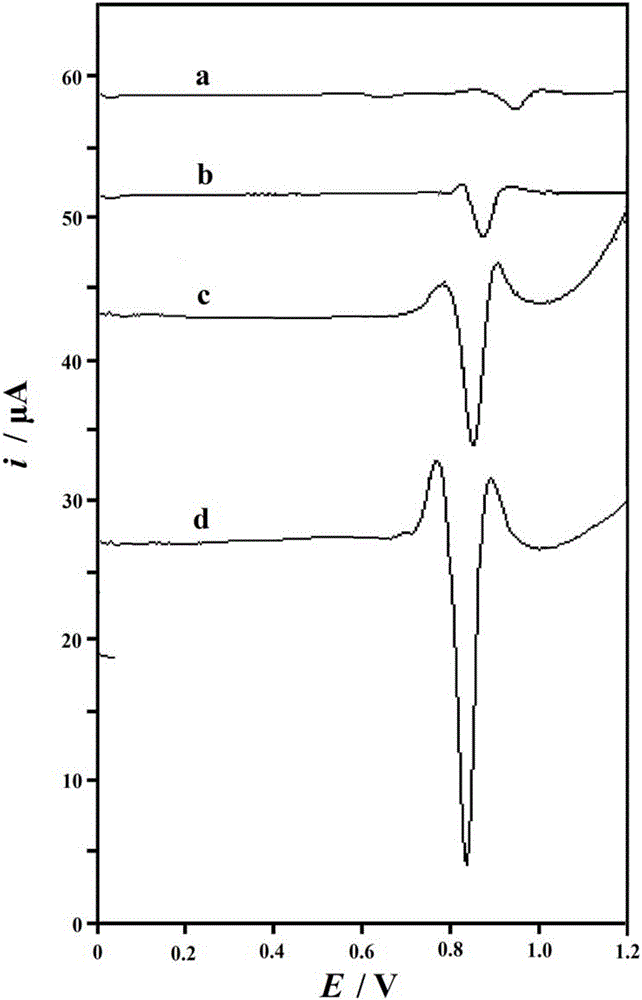
Graphene-cuprous oxide composite film modified acetylene black electrode and detection method for detection of vanillin in food
ActiveCN105973956AImprove wettabilityImprove surface activityMaterial electrochemical variablesComposite filmNanoparticle
The invention discloses a method for detection of vanillin in food, and belongs to the field of analytical chemistry or the field of food safety. With a graphene-supported cuprous oxide nanoparticle composite film modified acetylene black electrode as a working electrode, the vanillin in the food is detected by an electrochemical method. The detection method has the advantages of wide linear range, high sensitivity, low detection limit, simple operation, fast detection, low detection cost and accurate results.
Owner:HENGYANG NORMAL UNIV
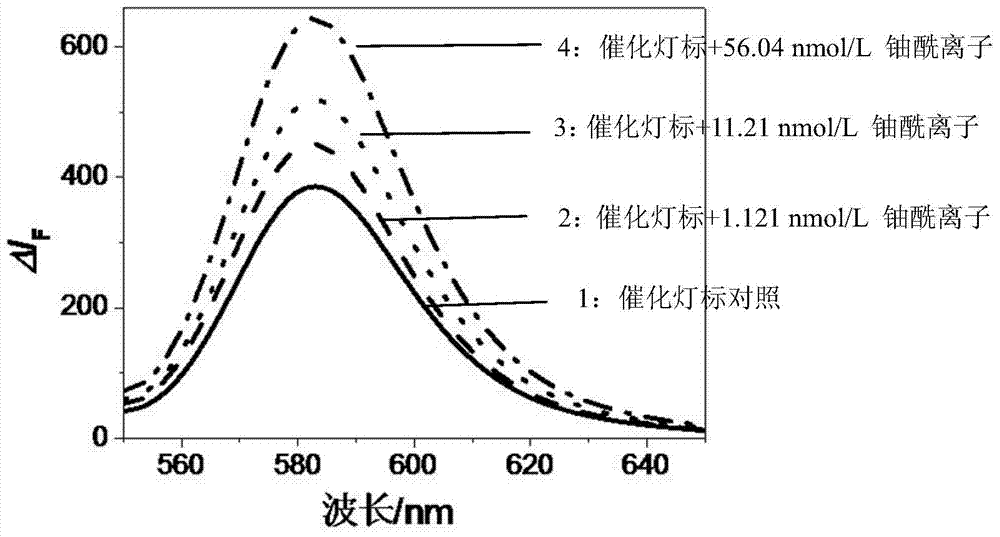
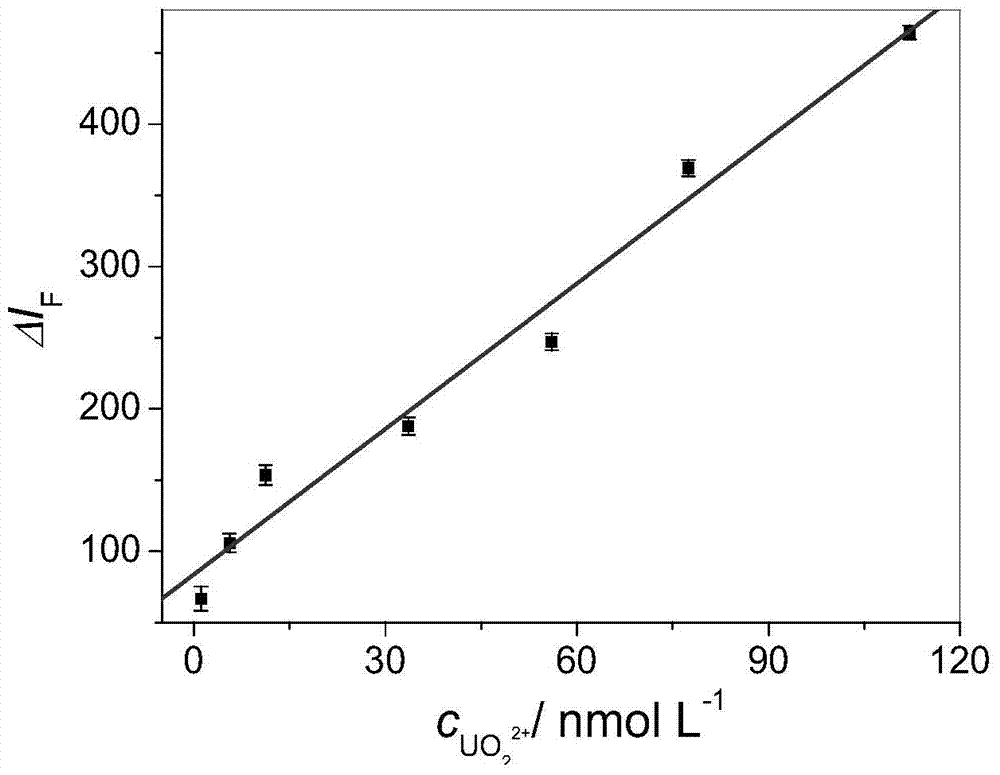
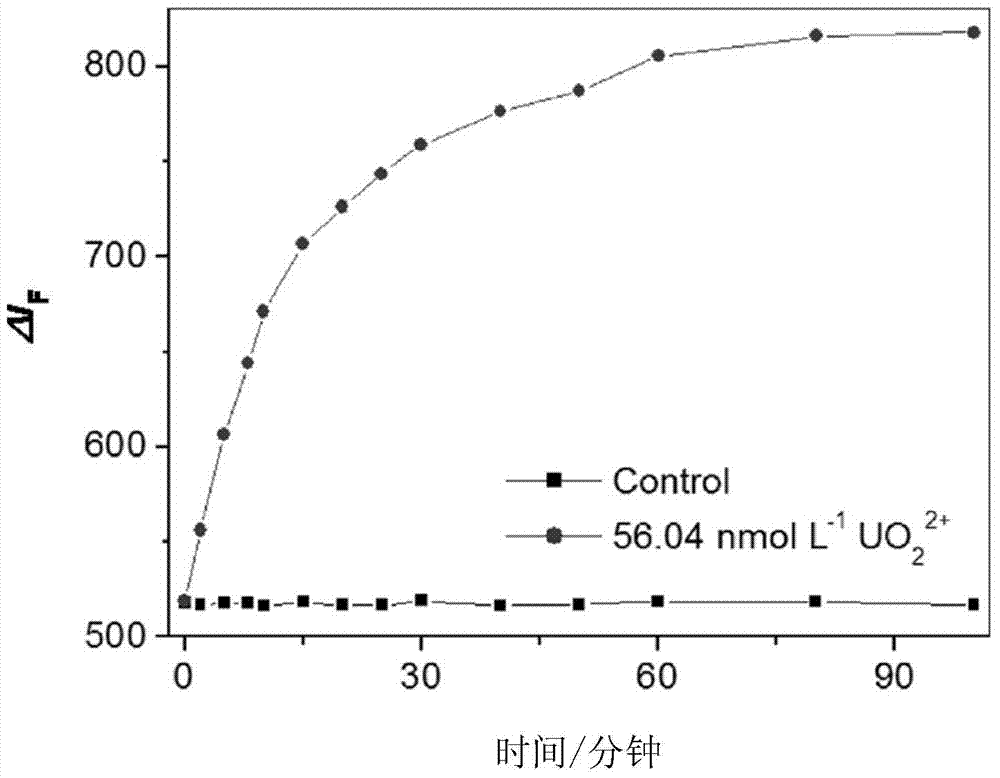
Catalytic light mark and preparation method thereof, and method for determination of trace uranium by catalytic light mark
InactiveCN104774915AThe detection process is fastImprove accuracyMicrobiological testing/measurementFluorescence/phosphorescenceMethyl groupUranyl nitrate
The invention discloses a catalytic light mark and a preparation method thereof, and a method for determination of trace uranium by the catalytic light mark. The catalytic light mark is composed of two parts of a DNA enzyme and a substrate DNA, a 3' end of a DNA enzyme sequence comprises four continuous guanine deoxynucleotides (G), the middle of a substrate DNA sequence has an adenine nucleotide (rA), and a 5' end of the substrate DNA is modified by a 4-methyl-6-carboxyl rhodamine (TAMRA) fluorescent dye. The DNA enzyme and the substrate DNA are in mixing hybridization to form the catalytic light mark, the catalytic light mark is added to a reaction system of a to-be-measured sample to undergo a reaction, the fluorescence intensity of uranyl nitrate ions with known concentration and the fluorescence intensity of the sample are determined, and the uranium content in the sample can be obtained through calculation. The method is a fast, accurate, high-sensitivity and high-selectivity method for determination of trace uranium.
Owner:EAST CHINA UNIV OF TECH
Method for detecting residual solvents in heparin sodium by headspace gas chromatography
InactiveCN108318615AOvercoming technical problems of poor applicabilitySuitability meets requirementsComponent separationRelative standard deviationPeak area
The invention relates to the technical field of solvent residual detection of drugs, and concretely relates to a method for detecting residual solvents in heparin sodium by headspace gas chromatography. The method optimizes the headspace sampling balance temperature which is an important parameter affecting the peak area ratio of the analytes to an internal standard by adopting the headspace gas chromatography against the technical problem of poor system adaptability of methods for detecting the residual solvents in the heparin sodium in the prior art, and the headspace balance temperature isoptimized to 78-82 DEG C from 90 DEG C. The method for detecting residual solvents in heparin sodium by headspace gas chromatography allows the relative standard deviation of the peak area ratio of the analytes to the internal standard during the repeated introduction of a control to be less than 1.0% and the system applicability to meet requirements, has the advantages of good specificity, good resolution of the analytes, good repeatability and high sensitivity, the detection limit of methanol is 0.0008%, the detection limit of ethanol is 0.0002%, the detection limit of acetone is 0.0001%, the quantitation limit of methanol is 0.0023%, the quantitation limit of ethanol is 0.0007%, and the quantitation limit of acetone is 0.0003%.
Owner:HUBEI YINUORUI BIOLOGICAL PHARMA
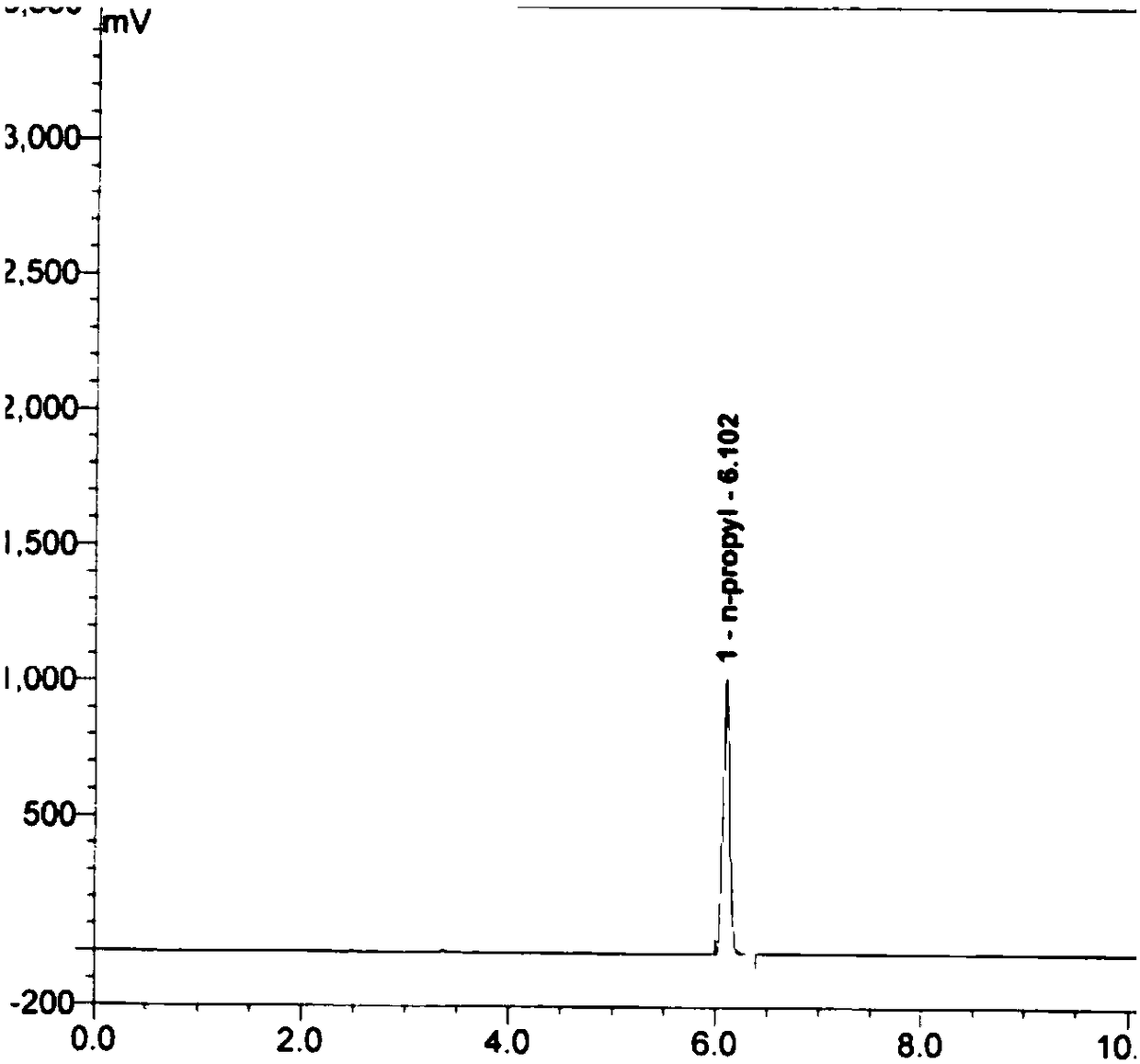
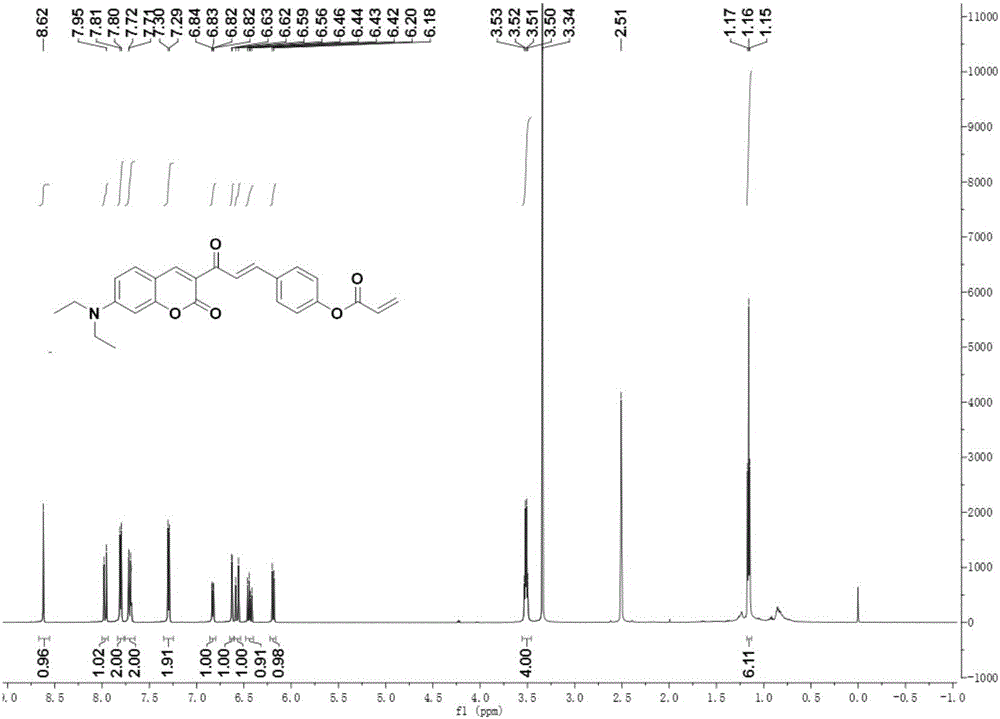
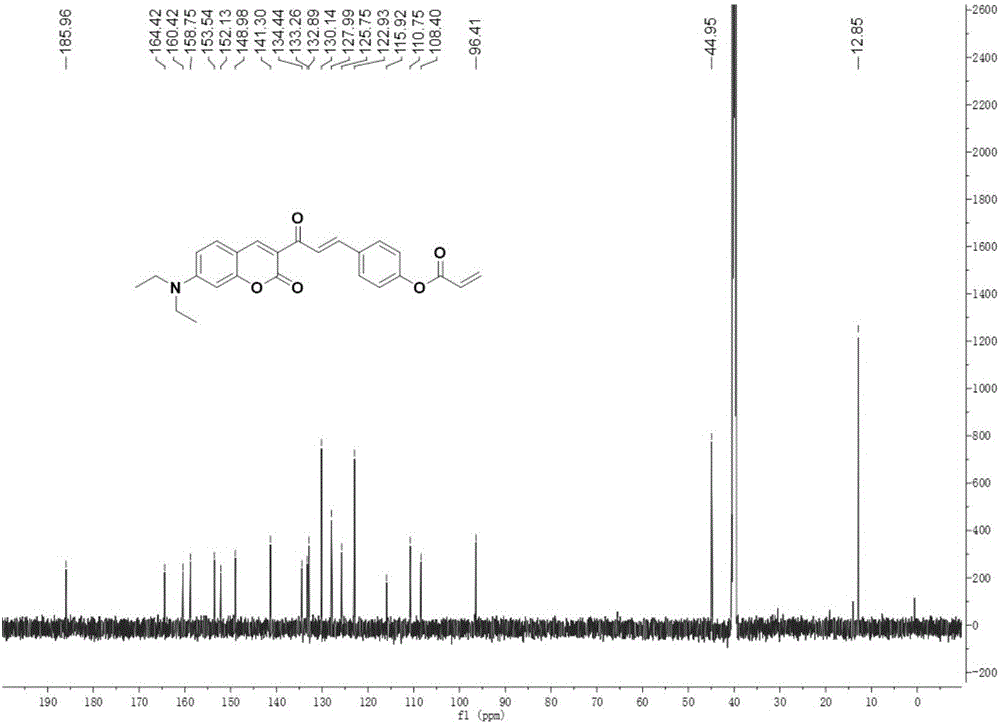
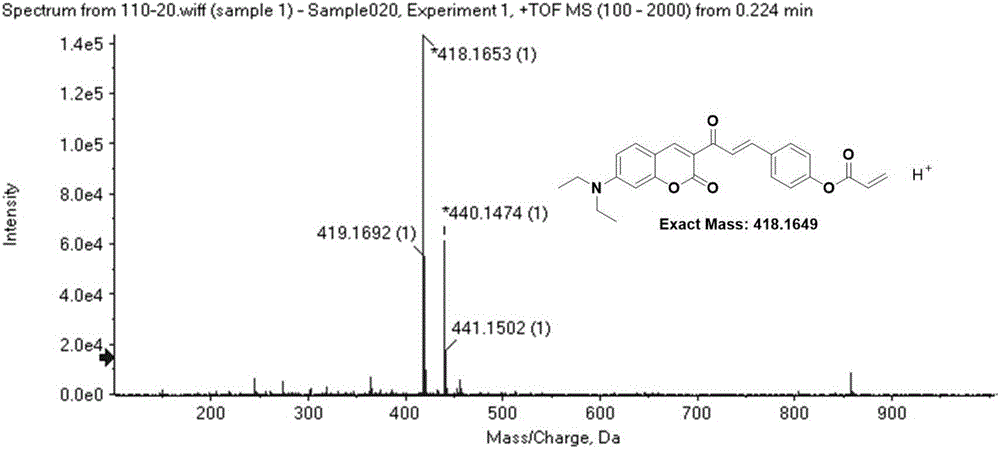
Coumarin derivative DOCOPA as well as preparation method and application thereof
InactiveCN106432164AIncreased sensitivityGood choiceOrganic chemistryFluorescence/phosphorescenceEvaporationAcryloyl chloride
The invention provides a coumarin derivative DOCOPA as well as a preparation method and an application thereof. The English name of DOCOPA is (E)-4-(3-(7-(diethylamino)-2-oxo-2Hchromen-3-yl)-3-oxoprop-1-en-1-yl) phenylacrylate). The preparation method comprises the steps that 3-acetyl-7-diethylaminocoumarin and p-hydroxybenzaldehyde are dissolved in acetonitrile firstly, a catalytic amount of piperidine is added, and reflux is performed until a reaction is performed sufficiently; an intermediate compound is further obtained; the intermediate compound and triethylamine are dissolved in dichloromethane, acryloyl chloride is added dropwise gradually to an ice bath, room-temperature stirring is performed until a reaction is performed sufficiently, a product is subjected to column chromatography separation after reduced-pressure evaporation, and the DOCOPA is obtained. The DOCOPA is applied to distinguishing and detection of cysteine and homocysteine in a pH7.8 system and imaging of the cysteine and the homocysteine in cells. The DOCOPA shows high sensitivity and selectivity for the cysteine and the homocysteine.
Owner:SHANXI UNIV
Cysteine detection method
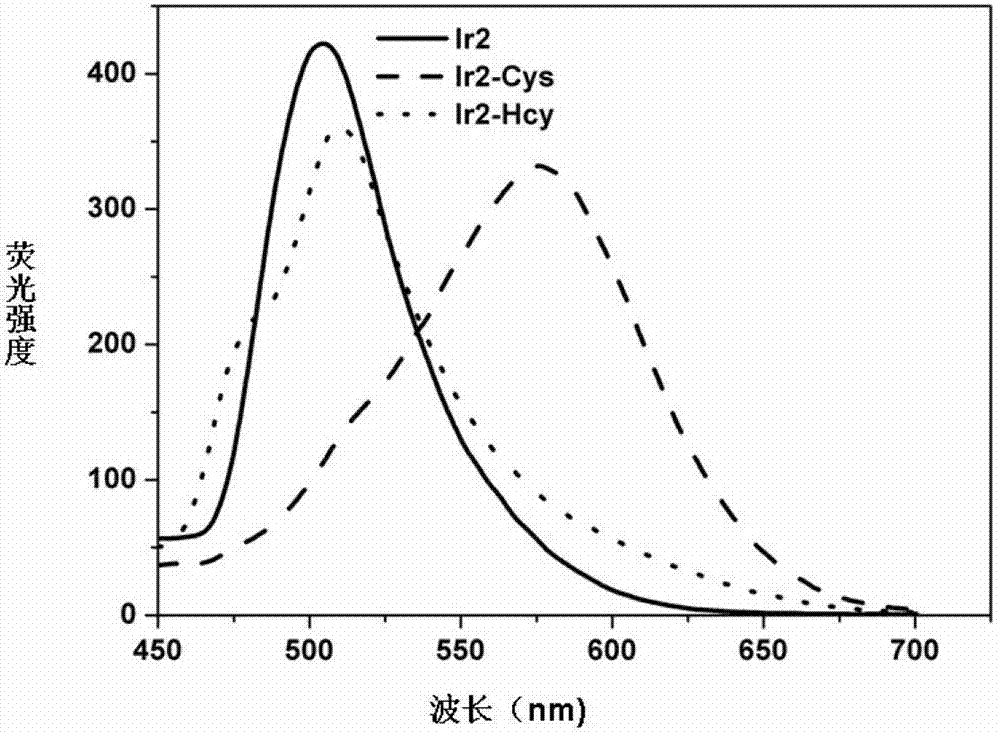
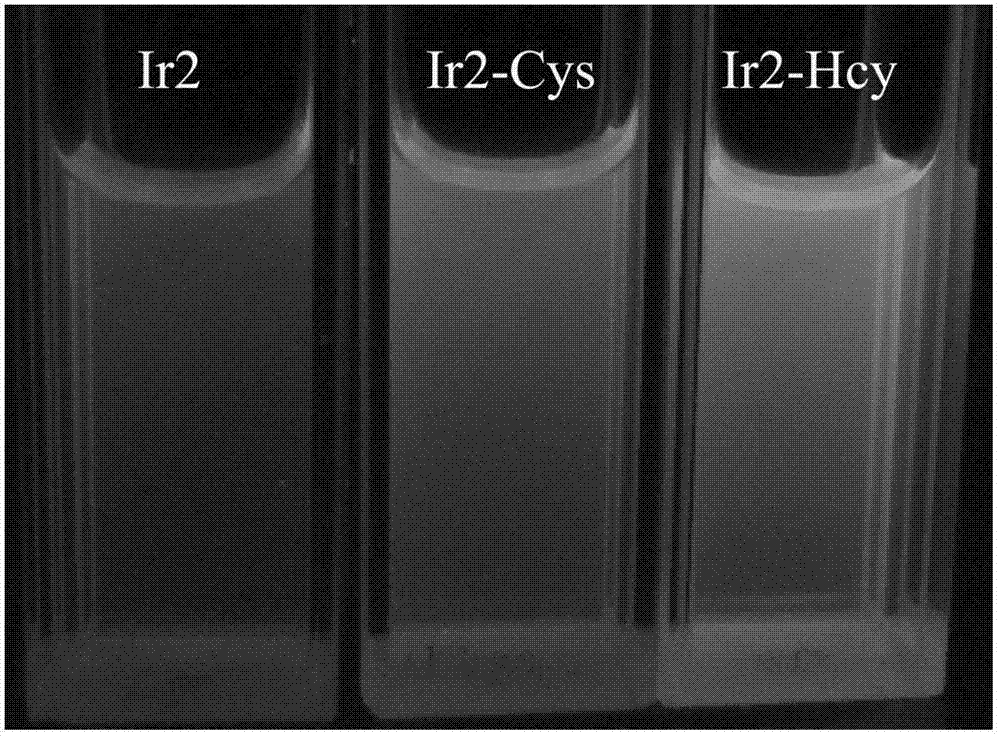
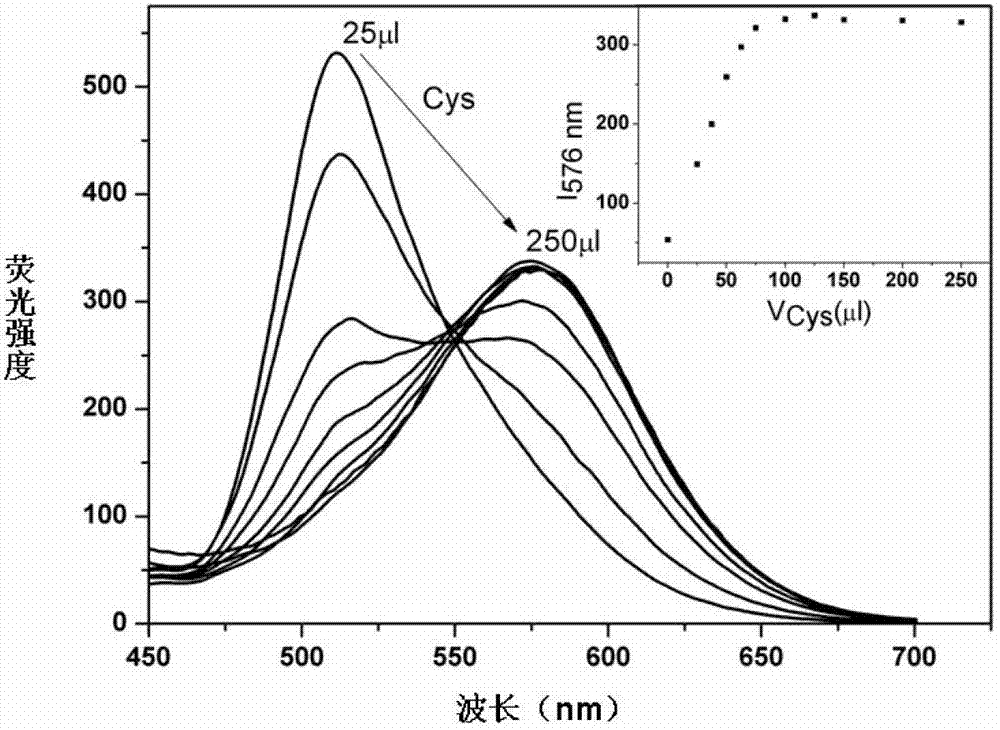
InactiveCN102928392ADo not interfere with determinationReduce testing costsFluorescence/phosphorescenceFluorescenceAqueous solution
The invention provides a method capable of detecting cysteine in a half-aqueous solution. Importantly, the method can distinguish cysteine from homocysteine. Based on a complex dichlorotetra[2-(2-pyridyl)phenyl]diiridium(III)[Ir2(ppy)4Cl2], in the half-aqueous solution of DMF (dimethylformamide) or DMSO (dimethylsulfoxide), the cysteine is directly detected by a fluorescent method. The determination process is simple and convenient and free from the interference of other amino acids including homocysteine and anions in the solution; and the method can be used for detecting whether cysteine exists or not.
Owner:SHANXI UNIV
Method for determining silicone oil in cosmetics
InactiveCN102944529AMethod instrument commonLow costColor/spectral properties measurementsFourier transform infrared spectroscopyTest sample
The invention discloses a method for determining silicone oil in cosmetics, which comprises the following steps that a test sample is dispersed with water in a color comparison tube; after being dispersed, the test sample is transferred to a separating funnel with water to be fully shaken; silicone oil in the test sample is extracted with normal hexane; after standing, moisture is removed through filtering, and a filtrate is obtained; and then the content of the silicone oil in the filtrate is determined by using the Fourier transform infrared spectroscopy. According to the method, the test sample matrix is dispersed with the water, the silicone oil is fully extracted with the normal hexane, the pretreatment is easy and quick, the dissolving capacity of the normal hexane to the silicone oil is strong, and the self infrared light does not interfere with the quantification of the silicone oil.
Owner:PONY TESTING INT GRP SHANGHAI CO LTD
Method for detecting content of heavy metals
InactiveCN107764818ADo not interfere with determinationGood colorMaterial analysis by observing effect on chemical indicatorPotassium iodineAqueous solution
The invention provides a method for detecting a content of heavy metals, which belongs to the technical field of the detection of metal ions. The method comprises the following steps: (1) preparing asolution to be detected: preparing a heavy metal sample to be detected into an aqueous solution, adding an acidic solution and a potassium iodide solution, and obtaining the solution to be detected; (2) detecting the solution to be detected: moving the solution to be detected, dropwise adding the solution to be detected onto test paper, developing the color for 50s to 100s, wherein the test papercontains dye; and (3) judging a detection result: visually comparing the color developing test paper and a colourimetric card, and determining a concentration of the heavy metal. By adopting the method for detecting the heavy metals, a rapid detection effect can be achieved, the detection efficiency is increased, and the field operation is also facilitated.
Owner:HEZHOU UNIV
Super fast liquid phase chromatography-tandem mass spectrometric method for determining hydroxypropyl-beta-cyclodextrin
InactiveCN101685083ACheck stabilityStrong specificityComponent separationMaterial analysis by electric/magnetic meansTandem mass spectrometryLiquid phase
The invention relates to a super fast liquid phase chromatography-tandem mass spectrometric method for determining hydroxypropyl-beta-cyclodextrin. The method comprises the following steps: (1) preparing hydroxypropyl-beta-cyclodextrin standard curve solution; (2) preparing hydroxypropyl-beta-cyclodextrin quality control solution; (3) treating a sample; and (4) performing liquid phase chromatography-tandem mass spectrometric analysis on the standard curve solution and the quality control solution respectively, and establishing a method for determining the hydroxypropyl-beta-cyclodextrin in thesample solution. The method has simple operation and good reproducibility, and can quantitatively analyze HP-beta-CD.
Owner:贾正平
Ultraviolet absorption measurement method for waste gas sulfur dioxide of stationary pollution source
ActiveCN104406932AEliminate or reduce pollutionEliminate or reduce adsorptionColor/spectral properties measurementsUltraviolet absorptionLength wave
The invention discloses an ultraviolet absorption measurement method for waste gas sulfur dioxide of a stationary pollution source. According to the method, an ultraviolet absorption method sulfur dioxide analyzer or multiple groups of gas analyzers with an ultraviolet absorption method sulfur dioxide analysis function is / are used as multiple monitors; sulfur dioxide is used for absorbing light with the characteristic wavelength of 240-330nm in a near ultraviolet light region, and the concentration of sulfur dioxide in waste gas is quantified according to the lambert-beer law; then the sulfur dioxide discharging rate is obtained by further calculation. An ultraviolet absorption method can adapt to wider measurement on SO2 discharged by the stationary source; by the method, the requirements on supervising monitoring on an on-site pollution source, comparison monitoring on a CEMS (continuous emission monitoring system) and checking of data effectiveness are met.
Owner:山东省环境监测中心站
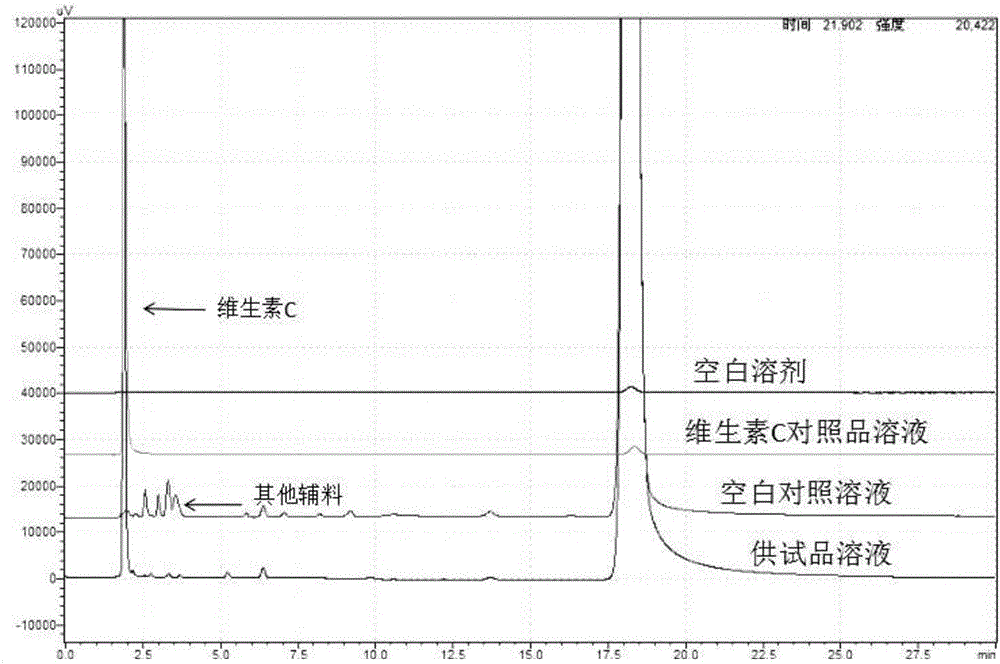
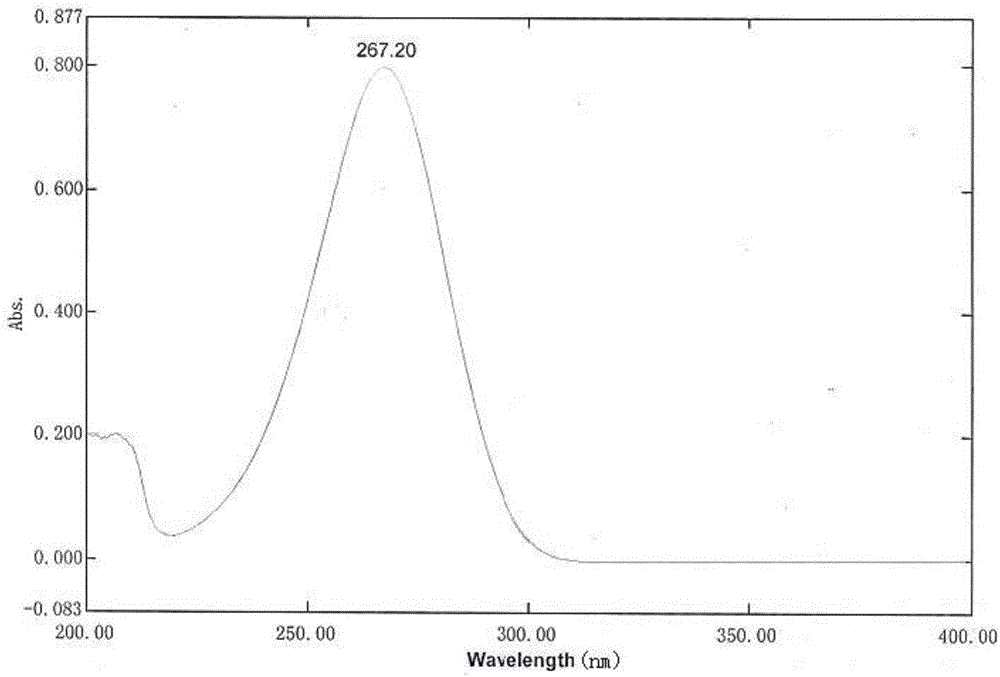
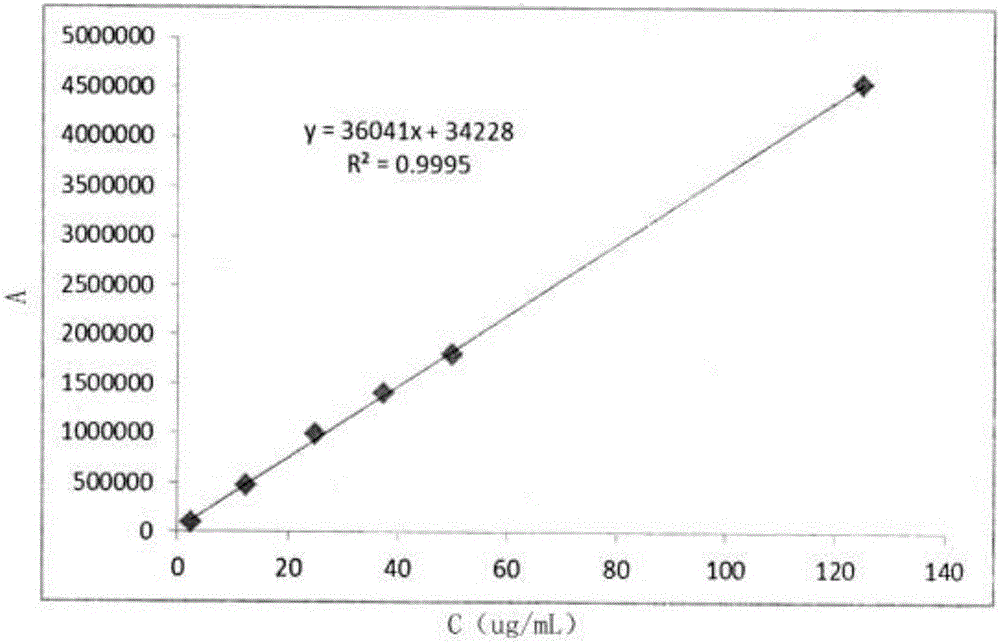
Detection method of content of vitamin C in vinpocetine injection
InactiveCN106645527AEnsure safetyThe detection method is simpleComponent separationVitamin CLinear regression
The invention discloses a detection method of the content of vitamin C in a vinpocetine injection. The detection method comprises the following steps: carrying out spectrum scanning through an ultraviolet-visible spectrophotometer to determine the maximum absorption wavelength lambdamax of a vitamin C standard solution; then preparing one group of vitamin C solutions with different concentrations and determining a chromatographic peak area of each vitamin C solution through a high performance liquid chromatograph under the maximum absorption wavelength lambdamax; drawing a standard curve of the vitamin C solutions and the corresponding chromatographic peak areas and solving a linear regression equation; meanwhile, determining a chromatographic peak area of a test sample solution through the high performance liquid chromatograph under the maximum absorption wavelength lambdamax; and finally, calculating the content of vitamin C in the test sample solution. According to the detection method disclosed by the invention, the content of vitamin C in the vinpocetine injection is detected by adopting an HPLC (High Performance Liquid Chromatography) method; and the detection method is simple, convenient and feasible, has high specificity and high accuracy and provides guarantees for safety, effectiveness and controllability of drugs.
Owner:武汉华龙生物制药有限公司
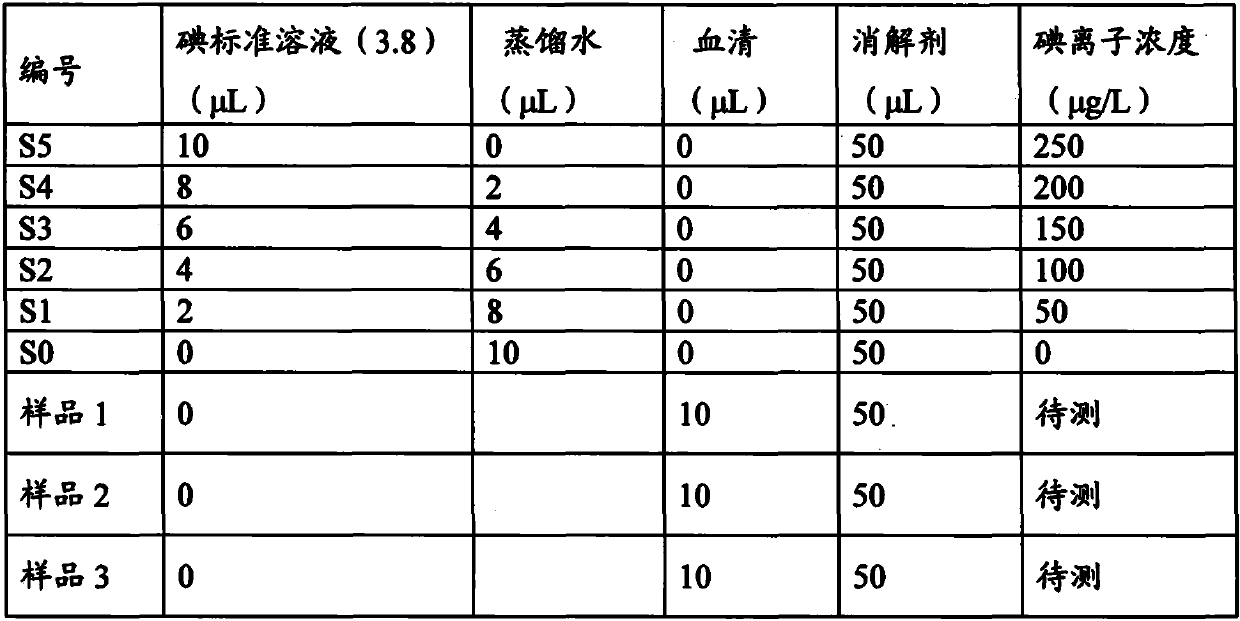
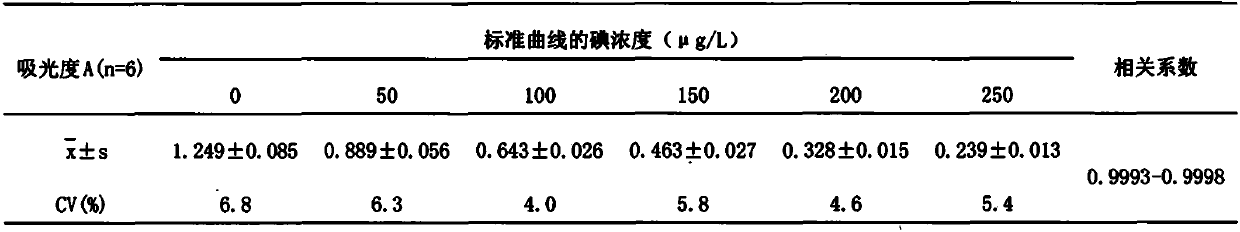
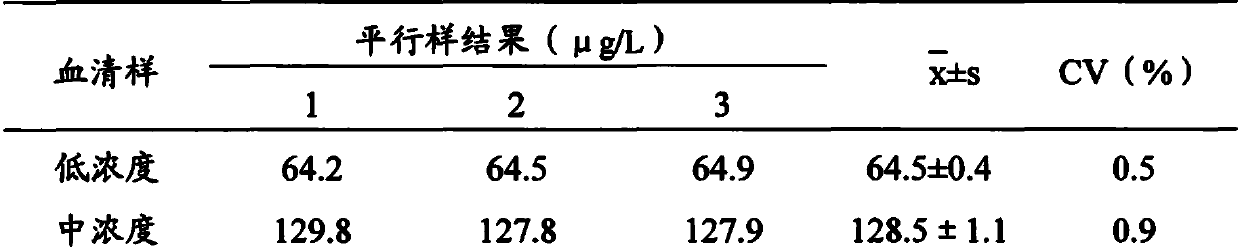
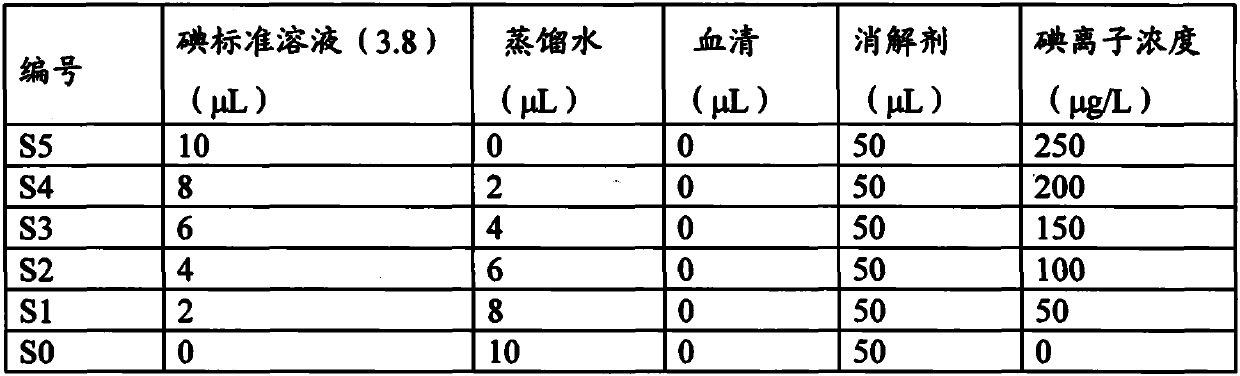
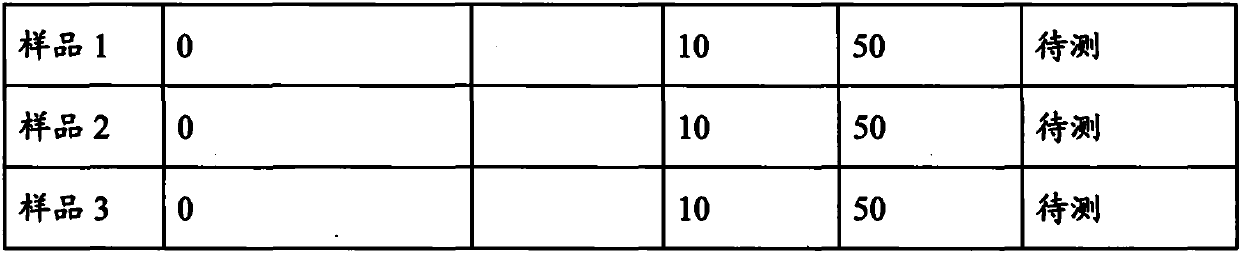
Arsenic-free detection method of iodide ions in trace serum sample for individual iodine nutrition evaluation
PendingCN111122463ADo not interfere with determinationAccurate measurementPreparing sample for investigationColor/spectral properties measurementsIodine deficiencyNutrition
The invention discloses an arsenic-free detection method of iodide ions in a trace serum sample for individual iodine nutrition evaluation. The arsenic-free detection method overcomes the defect thata highly toxic reagent is used in an existing method, adopts a sodium chlorate-sulfuric acid solution for digesting the serum sample at the temperature of 110 DEG C, uses iodine catalyzing the reduction reaction of sodium nitrite and ferric thiocyanate, and measures a concentration change speed of Fe(SCN)6<3-> to be in direct proportion to the iodine content; the reaction temperature and the reaction time are precisely controlled, the absorbance of the remaining Fe(SCN)6<3-> is measured, and the iodine content and the logarithm of the absorbance are in a linear relation. The establishment of the arsenic-free detection method can further meet the gradually increased demand of people for knowing the iodine nutrition level, a powerful method and means are added to serum iodine detection of iodine deficiency disease prevention and treatment monitoring and iodine nutrition evaluation, and the arsenic-free detection method has important public health practical significance and practical application value.
Owner:HARBIN MEDICAL UNIVERSITY
Arsenic-free detection kit for iodide ions in trace serum sample
PendingCN111122465AEasy to operateEasy to carryPreparing sample for investigationColor/spectral properties measurementsSodium chlorateSerum samples
The invention discloses an arsenic-free detection kit for iodide ions in a trace serum sample. According to the arsenic-free detection kit, the defect that a highly toxic reagent is used in the priorart is overcome, a sodium chlorate-sulfuric acid solution is used for digesting a serum sample at 110 DEG C, iodine is used for catalyzing the reduction reaction of sodium nitrite and ferric thiocyanate, and the concentration change speed of Fe(SCN)6<3-> is measured to be in direct proportion to the iodine content; the reaction temperature and the reaction time are precisely controlled, the absorbance of the remaining Fe(SCN)6<3-> is measured, and the iodine content and the logarithm of the absorbance are in a linear relation. An existing serum iodine quantitative determination method has thedefects of complex reagent preparation, easiness in pollution and decomposition, large dosage of dangerous reagents and large required serum amount. The arsenic-free serum iodine detection kit researched by the invention is convenient to operate, easy to carry, high in safety and suitable for clinically developing individual iodine nutrition detection.
Owner:HARBIN MEDICAL UNIVERSITY
Method for sensitively detecting tannin acid in beer
InactiveCN110243796ADo not interfere with determinationImprove anti-interference abilityFluorescence/phosphorescenceFluorescenceQuenching
The present invention relates to a method for sensitively detecting tannin acid (TA) in beer. The method comprises the steps of: taking graphene oxide (GO) and ammonia water (NH3.H2O) as raw materials, and performing hydrothermal cutting under a strong alkaline condition to prepare amination GQDs (AF-GQDs). The polyphenolic hydroxyl structure of TA enables hydrogen bonding between the TA and the AF-GQDs, and a strong pi-pi interaction can be generated, so that fluorescence quenching of AF-GQDs is caused. A TA quantitative analysis method is established by using the AF-GQDs as a fluorescent probe, and the detection limit is 43.3 nmol / L<-1>; and the method has strong anti-interference ability, and detection of the TA is not interfered by the common small molecules, metal ions, anions, and the like.
Owner:QINGDAO UNIV
Super fast liquid phase chromatography-tandem mass spectrometric method for determining hydroxypropyl-beta-cyclodextrin
InactiveCN101685083BCheck stabilityDo not interfere with determinationComponent separationMaterial analysis by electric/magnetic meansLiquid phaseChemistry
The invention relates to a super fast liquid phase chromatography-tandem mass spectrometric method for determining hydroxypropyl-beta-cyclodextrin. The method comprises the following steps: (1) preparing hydroxypropyl-beta-cyclodextrin standard curve solution; (2) preparing hydroxypropyl-beta-cyclodextrin quality control solution; (3) treating a sample; and (4) performing liquid phase chromatography-tandem mass spectrometric analysis on the standard curve solution and the quality control solution respectively, and establishing a method for determining the hydroxypropyl-beta-cyclodextrin in the sample solution. The method has simple operation and good reproducibility, and can quantitatively analyze HP-beta-CD.
Owner:贾正平
Method for determining antimony content in antimony oxide master batch by cerous sulfate titration method
PendingCN114252438ALow costShorten detection timeChemical analysis using titrationMaterial analysis by observing effect on chemical indicatorCeric sulfateSulfate
The method comprises the following steps: (1) putting the antimony oxide master batch into a bottle, adding potassium sulfate and sulfuric acid, heating and dissolving on an electric furnace, taking down and slightly cooling, adding a reducing agent for reduction without shaking, and taking down and cooling when small bubbles in the solution disappear and thick white smoke is emitted to a bottle opening; (2) adding water along the bottle wall, uniformly shaking, heating and boiling, taking down and slightly cooling, adding hydrochloric acid, uniformly shaking, heating and taking down to prepare a heating solution; (3) adding an indicator into the heating liquid prepared in the step (2), uniformly shaking, keeping the temperature of the heating liquid, and dropwise adding a cerous sulfate standard titration solution until the red color of the solution just disappears, thereby obtaining an end point; and (4) calculating the mass fraction omega sb of antimony. The method is simple, reliable, convenient to operate and stable in result, can meet the requirement for analyzing the content of antimony in the antimony oxide master batch, and effectively solves the practical technical problem that the prior art is not suitable for detecting the total antimony in the antimony oxide master batch.
Owner:广西华锑科技有限公司
Method and device for detecting tripolycyanamide
ActiveCN101706469BReduce testing costsSuitable for online dynamic analysis and monitoringComponent separationMaterial analysis by electric/magnetic meansIon exchangePre treatment
The invention relates to the detection of tripolycyanamide, in particular to a method and a device for detecting tripolycyanamide. The method comprises: preprocessing a sample to be detected to remove proteins; allowing obtained clear liquid to pass through an anionic and cationic ion exchange series column at a constant flow rate; transferring the clear liquid to be detected and buffer solution into a cell for samples to be detected; inserting an ion selective electrode modified by a molecular imprinted polymer into the cell; generating an electric potential signal; and determining the tripolycyanamide content of the sample to be detected by using an electric potential value according to a standard curve. In the device, one end of the anionic and cationic ion exchange series column, a second flow injection device and a container for the samples to be detected are connected with a three-way valve respectively; the other end of the anionic and cationic ion exchange series column is connected with an ultramicropore protein filter through a first flow injection device; and the second flow injection device is connected with a buffer solution tank; and the ion selective electrode modified by the molecular imprinted polymer is inserted into the container for the samples to be detected. The method and the device have the advantages of high flexibility, low operation cost, suitabilityfor in-situ monitoring and the like in the detection of tripolycyanamide.
Owner:YANTAI INST OF COASTAL ZONE RES CHINESE ACAD OF SCI
Method for Separation and Detection of Acetylcysteine Enantiomers
ActiveCN109725073BIncrease mutual attractionHigh selectivityComponent separationEnantiomerAcetylcysteine
The invention discloses a method for separating and detecting acetylcysteine enantiomers. In an organic solvent, a derivatization reagent is used to carry out the separation and detection of N-acetyl-L-cysteine and its enantiomers. The derivatization reaction is to prepare derivatized products of N-acetyl-L-cysteine and its isomers, which are then separated and detected by normal phase high-performance liquid chromatography; the derivatization reagents are isocyanates. In the method for separating and detecting acetylcysteine enantiomers of the present invention, the derivatization reagent is reacted with N-acetyl-L-cysteine and its enantiomers, and pre-column derivatization is used for normal phase and high efficiency. Liquid chromatography, based on the mechanism of chiral separation, increases the mutual attraction between the stationary phase in the chromatographic column and the chiral substance to be separated, effectively improving the selectivity of chiral separation. The method is simple, time-consuming and easy to popularize and use.
Owner:HUNAN WARRANT PHARM TECH DEV CO LTD +1
Method for determining concentration of chartreusin in blood plasma by liquid chromatography-tandem mass spectrometry
ActiveCN111751469AThe pretreatment method is simpleSuitable for routine determinationComponent separationFluid phaseInternal standard
The invention discloses a method for determining the concentration of chartreusin in blood plasma through liquid chromatography-tandem mass spectrometry. The method comprises the following steps: separating chartreusin from a blood plasma sample by liquid chromatography, then establishing a standard curve by taking the concentration of chartreusin in a calibration standard sample as an X axis andthe peak area ratio of chartreusin to an internal standard substance in the calibration standard sample as a Y axis by utilizing a mass spectrum internal standard quantitative method, and calculatingthe concentration of chartreusin in the blood plasma. The method has the following advantages of: (1) strong specificity: the retention time of the chartreusin and the internal standard naringenin isabout 0.6min, and endogenous substances do not interfere with the determination of the chartreusin and the internal standard naringenin; (2) short analysis time: the detection time of each sample is 1min; (3) high sensitivity: the minimum quantification limit is 1ng.ML<-1>; and (4) the linear range of the blood plasma standard curve of the method is 1-1000ng.ML<-1>, and the intra-batch precision CV and the inter-batch precision CV are both less than 10%.
Owner:JIANGSU PROVINCIAL HOSPITAL OF TCM
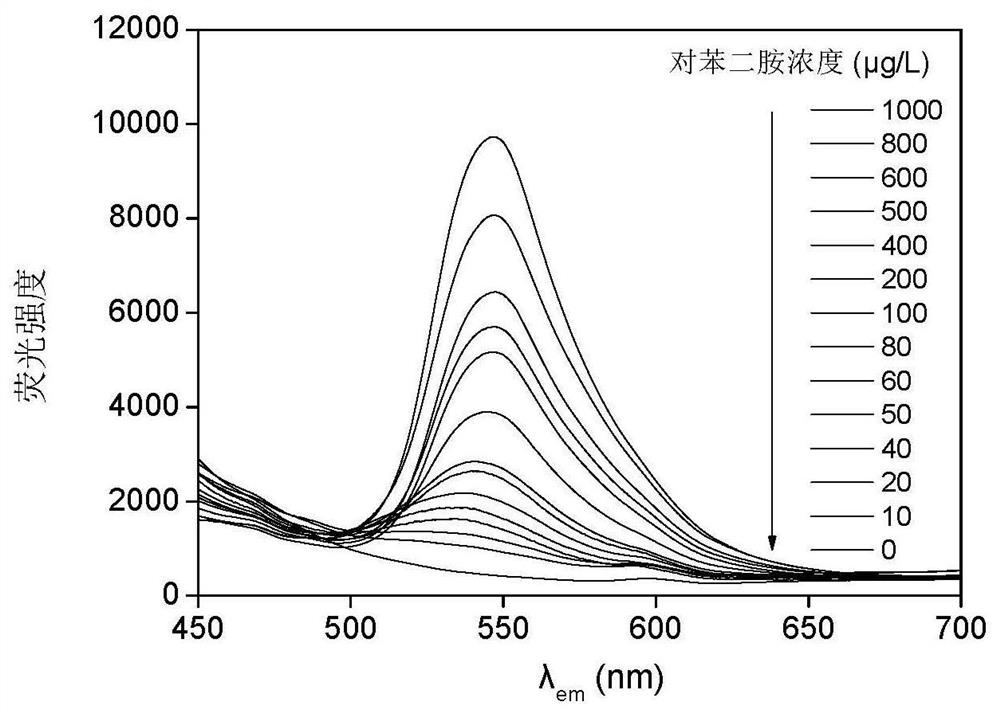
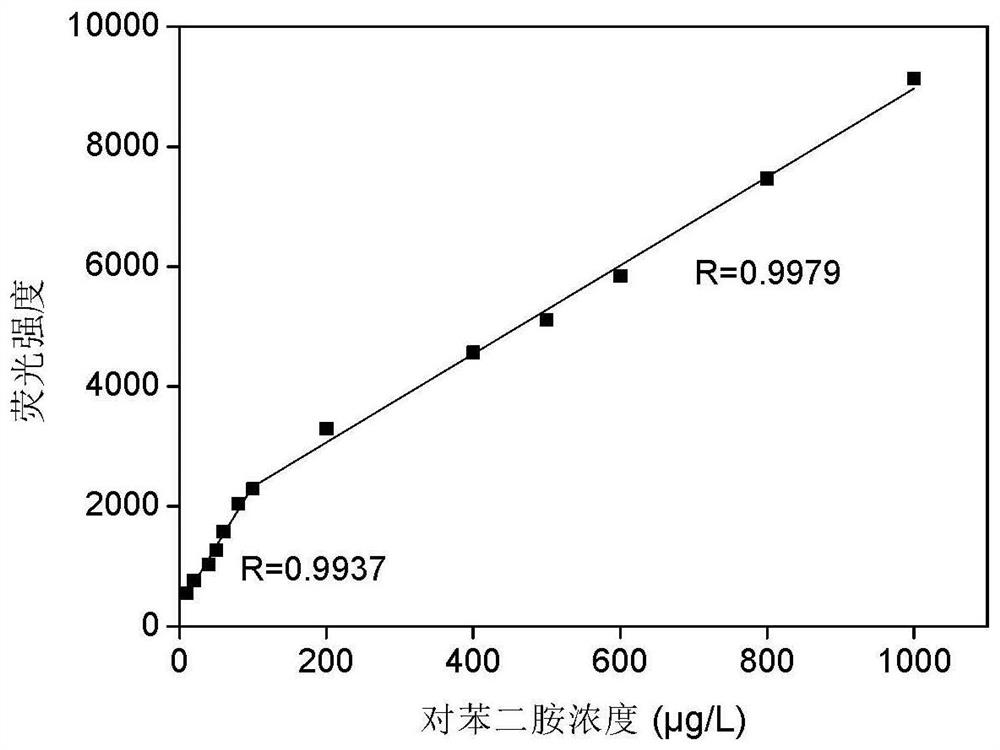
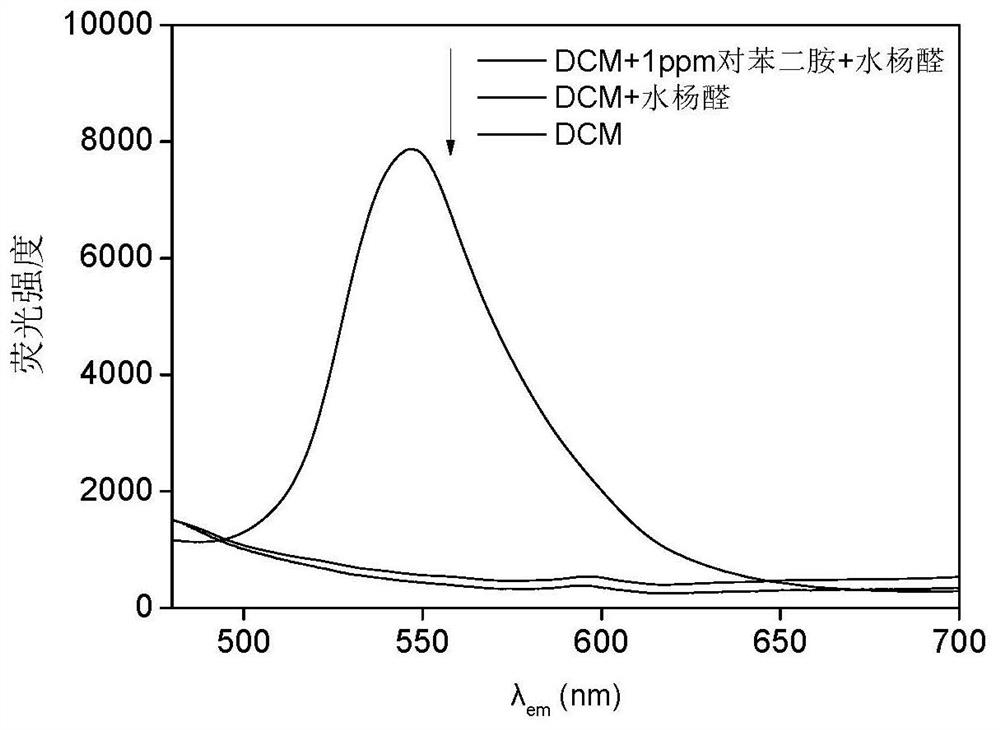
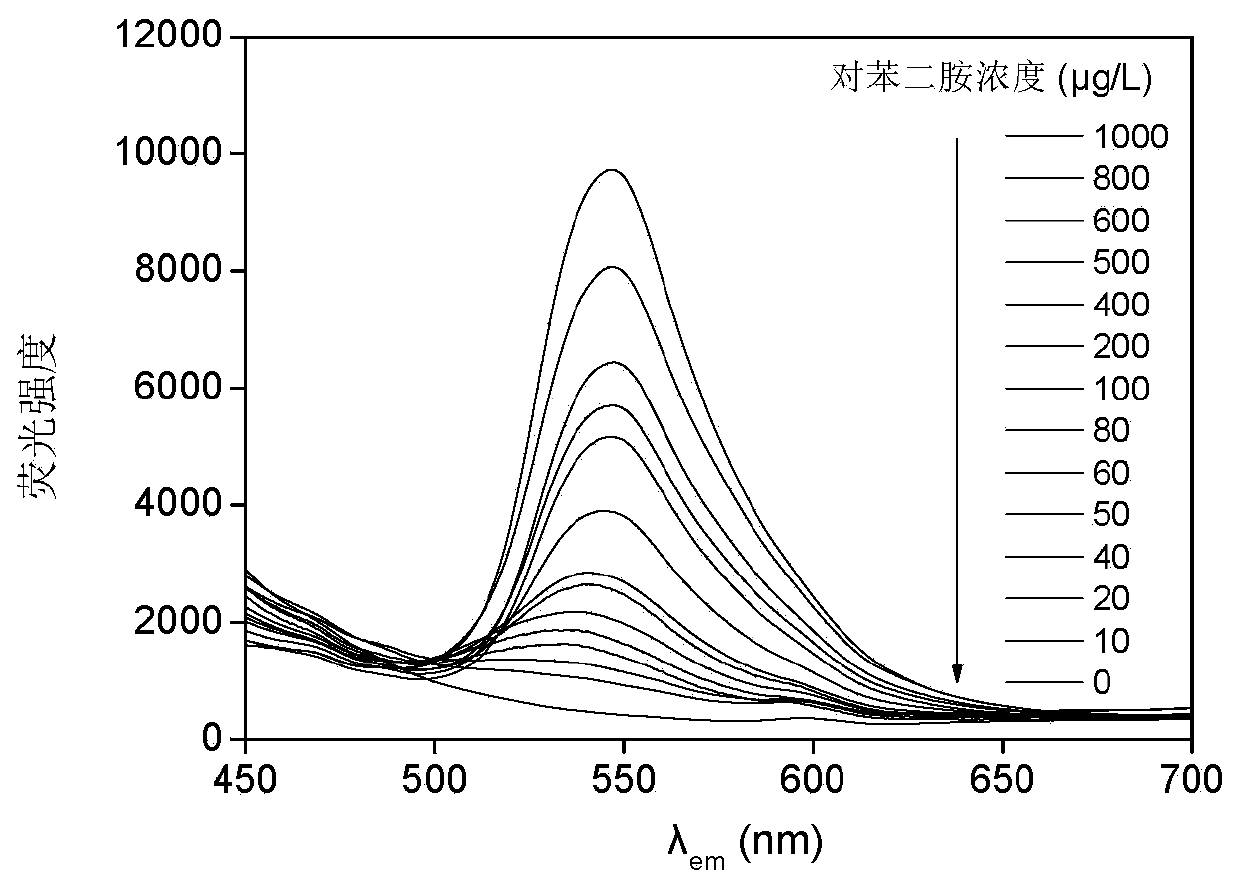
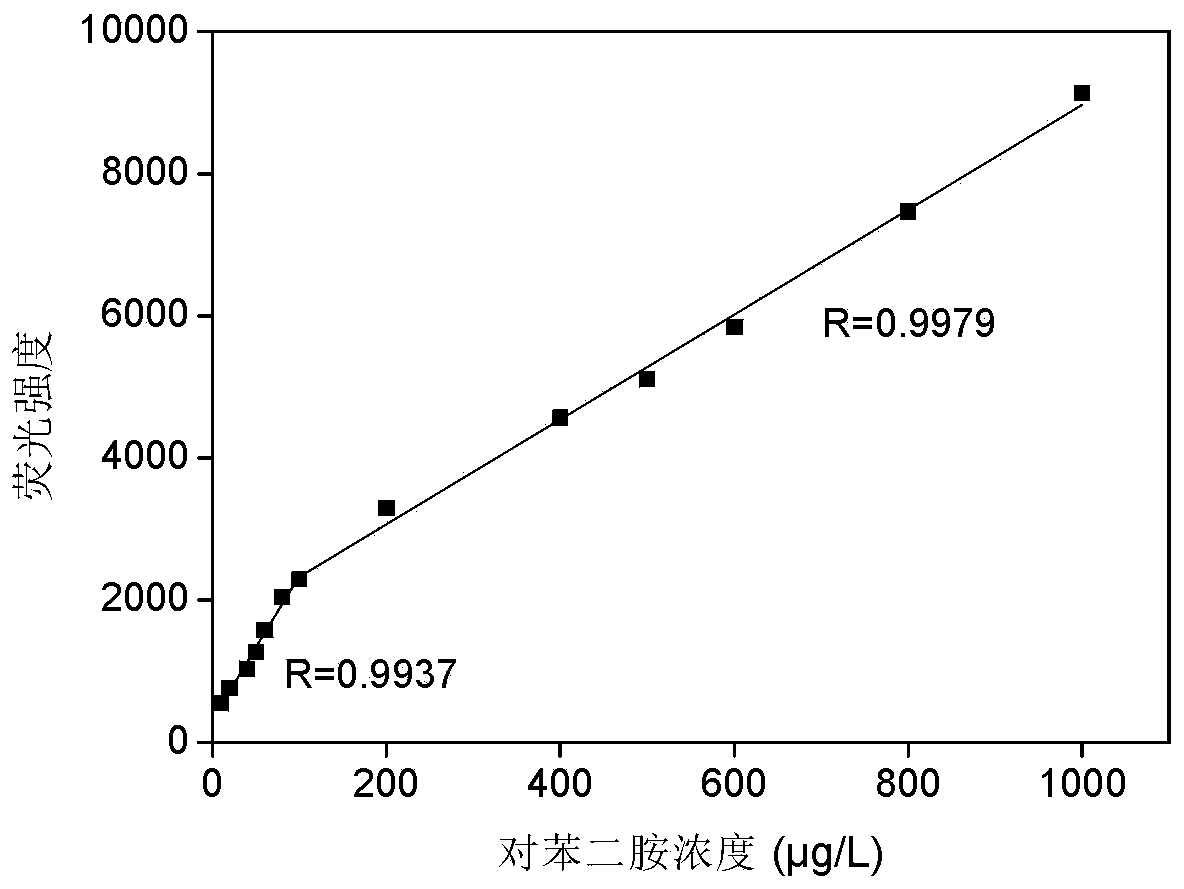
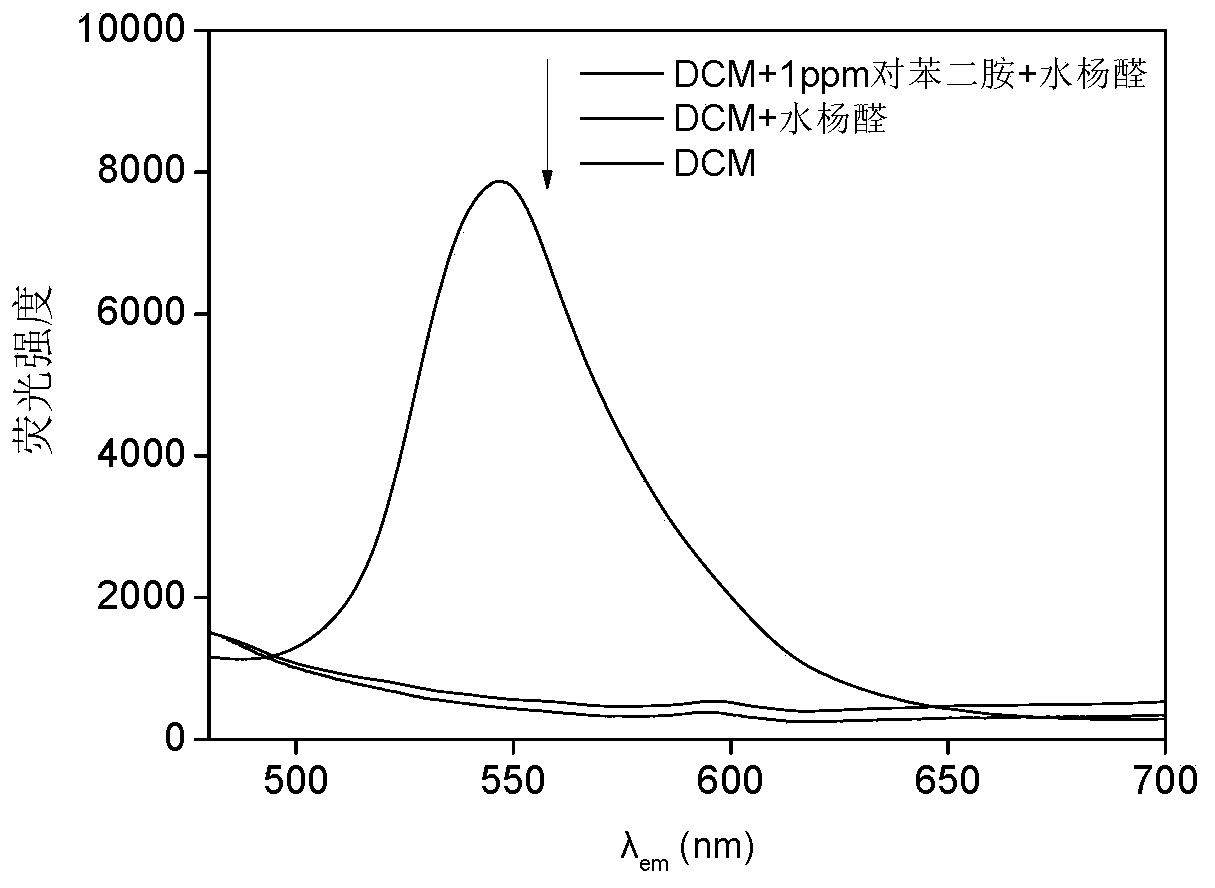
A fast and efficient method for detecting trace p-phenylenediamine
ActiveCN110530834BGood optical clarityHigh mechanical strengthFluorescence/phosphorescenceFluorescenceQualitative analysis
A method for quickly and efficiently detecting trace p-phenylenediamine, specifically, dissolving cotton cellulose to prepare a regenerated cellulose film, using sodium periodate to selectively oxidize the hydroxyl groups on the regenerated cellulose film to aldehyde groups, and then pass The two-step Schiff base reaction prepares solid-phase fluorescence detection membranes of various systems emitting yellow fluorescence, which are used for qualitative analysis and quantitative detection of p-phenylenediamine. The invention has the advantages of simplicity, rapidity, high sensitivity and good selectivity.
Owner:SOUTHWEST JIAOTONG UNIV
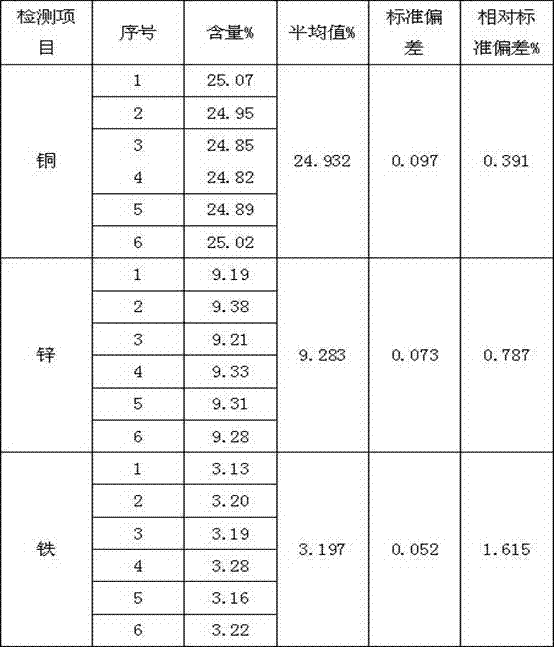
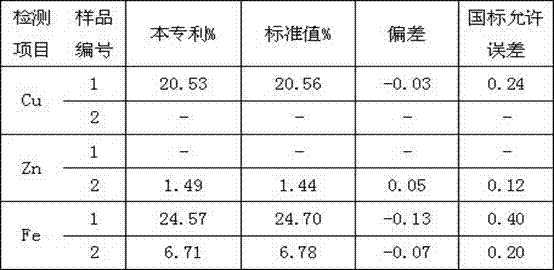
A Rapid Joint Measurement Method of Copper, Zinc and Iron Content in Gold Mud
ActiveCN105044088BFill vacancyDo not interfere with determinationMaterial analysis by observing effect on chemical indicatorSilver copperPrecipitation
Owner:山东黄金冶炼有限公司
A kind of coumarin derivative docopa and its preparation method and application
InactiveCN106432164BEasy to synthesizeLow costOrganic chemistryFluorescence/phosphorescenceEvaporationChemistry
Owner:SHANXI UNIV
Ultraviolet Absorption Determination Method for Sulfur Dioxide in Stationary Pollution Source Exhaust Gas
ActiveCN104406932BDo not interfere with determinationDetermination of a wide rangeColor/spectral properties measurementsLength waveNear ultraviolet
The invention discloses an ultraviolet absorption measurement method for waste gas sulfur dioxide of a stationary pollution source. According to the method, an ultraviolet absorption method sulfur dioxide analyzer or multiple groups of gas analyzers with an ultraviolet absorption method sulfur dioxide analysis function is / are used as multiple monitors; sulfur dioxide is used for absorbing light with the characteristic wavelength of 240-330nm in a near ultraviolet light region, and the concentration of sulfur dioxide in waste gas is quantified according to the lambert-beer law; then the sulfur dioxide discharging rate is obtained by further calculation. An ultraviolet absorption method can adapt to wider measurement on SO2 discharged by the stationary source; by the method, the requirements on supervising monitoring on an on-site pollution source, comparison monitoring on a CEMS (continuous emission monitoring system) and checking of data effectiveness are met.
Owner:山东省环境监测中心站
A kind of coumarin derivative ss-590 and its preparation method and application
ActiveCN113698413BEasy to synthesizeLow costOrganic chemistryFluorescence/phosphorescenceQuinoxalineBio molecules
Owner:SHANXI UNIV
Graphene-Cuprous Oxide Composite Film Modified Acetylene Black Electrode for Detecting Vanillin in Food and Its Detection Method
ActiveCN105973956BImprove wettabilityImprove surface activityMaterial electrochemical variablesComposite filmNanoparticle
Owner:HENGYANG NORMAL UNIV
Method for rapidly and efficiently detecting trace p-phenylenediamine
ActiveCN110530834AHigh optical clarityFluorescent background interference is smallFluorescence/phosphorescenceSolid phasesFluorescence
The invention discloses a method for rapidly and efficiently detecting trace p-phenylenediamine. The method specifically comprises the following steps of dissolving cotton cellulose to prepare a regenerated cellulose membrane; selectively oxidizing hydroxyls on the regenerated cellulose membrane into aldehyde groups by adopting sodium periodate; and carrying out a two-step Schiff base reaction toprepare a solid-phase fluorescence detection membrane of each system for emitting yellow fluorescence, wherein the solid-phase fluorescence detection membrane is used for qualitative analysis and quantitative detection of the phenylenediamine. The method has the advantages of simplicity and quickness, high sensitivity and good selectivity.
Owner:SOUTHWEST JIAOTONG UNIV
Quantitative analysis method for detecting blood concentration of YG-18 in rat plasma by liquid chromatography-mass spectrometry
PendingCN113866315AFew samplesEasy pretreatmentComponent separationChromatographic separationGradient elution
The invention relates to a quantitative analysis method for detecting the blood concentration of rat plasma YG-18 by liquid chromatography-mass spectrometry, which belongs to the technical field of pharmacokinetics preclinical pharmacokinetics, and comprises the following steps: sequentially adding acetonitrile and an internal standard working solution into SD rat plasma after YG-18 administration, carrying out vortex centrifugation, taking supernatant, dissolving the supernatant with a mobile phase, and loading the sample, wherein an internal standard working solution is an acetonitrile solution of etophenanil; then carrying out quantitative analysis, wherein a liquid chromatography-mass spectrometry technology is adopted, an acetonitrile-formic acid solution is used as a mobile phase for gradient elution, and an Alltima C18 chromatographic column is used for the chromatographic separation. The method is strong in specificity, high in sensitivity, small in sample sampling amount, simple and rapid in pretreatment, short in analysis period, accurate and reliable through methodology verification, and suitable for YG-18 drug concentration determination and pharmacokinetic research in SD rat plasma.
Owner:DALIAN MEDICAL UNIVERSITY
A method for detecting plasma concentration of nmda receptor antagonist jcc-02 based on hplc-ms/ms technology
ActiveCN108918722BFew samplesEasy pretreatmentComponent separationNR1 NMDA receptorChromatographic separation
The present invention provides a kind of method based on HPLC-MS / MS technology detection NMDA receptor antagonist JCC-02 blood concentration, relates to the field of drug analysis, comprising the following steps: SD rats after JCC-02 intragastric administration In the blood plasma, add methanol, acetonitrile and internal standard working solution in sequence, vortex to take the supernatant and dissolve it with the mobile phase and then inject the sample. The internal standard working solution is the methanol solution of gliclazide; ‑The mixed solution of formic acid and water is used as the mobile phase for gradient elution, the Venusil ASB C8 chromatographic column is used for chromatographic separation, and the secondary mass spectrometer is used for detection and quantitative analysis. The method has strong specificity, high sensitivity, less sample volume, simple and rapid pretreatment, and short analysis cycle. The method is verified by methodology to be accurate and reliable, and is suitable for the determination of drug concentration and drug concentration of JCC‑02 in SD rat plasma. Kinetic studies.
Owner:THE FIRST HOSPITAL OF CHINA MEDICIAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com