Method for detecting residual solvents in heparin sodium by headspace gas chromatography
A technology of headspace gas chromatography and residual solvents, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of poor applicability, achieve high sensitivity, high separation, and overcome the effects of poor system applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] The invention provides a method for detecting residual solvents in heparin sodium by headspace gas chromatography, the method comprising the following steps:
[0070] 1. Prepare the solution
[0071] (1) Preparation of internal standard solution: add 150ml of ultrapure water to a 250ml volumetric flask, then precisely weigh 25μl of n-propanol (density 0.80g / ml) into the 250ml volumetric flask, dilute with water to the mark, and shake well to prepare 80μg / ml n-propanol solution;
[0072] (2) Preparation of the test solution: Accurately weigh 2.0g of heparin sodium sample into a 10ml volumetric flask, add internal standard solution to dissolve and dilute to the mark, shake well; accurately pipette 3.0ml of the solution, place In the headspace bottle of sodium chloride 500mg, seal, be mixed with need testing solution; Repeat preparation 2 parts, as need testing solution 1, need testing solution 2.
[0073] (3) Preparation of mixed reference substance stock solution: add ...
Embodiment 2
[0101] Embodiment 2: specificity experiment
[0102] 1. Solution preparation
[0103] Blank solution: Accurately measure 3.0ml of water, put it in a headspace bottle pre-added with 500mg of sodium chloride, and seal it.
[0104] Preparation of internal standard solution: Add 150ml of ultrapure water into a 250ml volumetric flask, then precisely weigh 25μl of n-propanol (density 0.80g / ml) into the 250ml volumetric flask, dilute to the mark with water, shake well to prepare 80μg / ml n-propanol solution;
[0105] n-propanol reference substance solution: Accurately measure 3.0ml of internal standard solution, put it in a headspace bottle pre-added with 500mg of sodium chloride, and seal it.
[0106] Methanol reference substance solution: Accurately weigh 0.04g of methanol, put it into a 100ml volumetric flask with about 30ml of water, add water to dilute to the mark, shake well, take 3.0ml of this solution, put it into a headspace bottle with 500mg of sodium chloride added in adv...
Embodiment 3
[0117] Embodiment 3: System suitability experiment
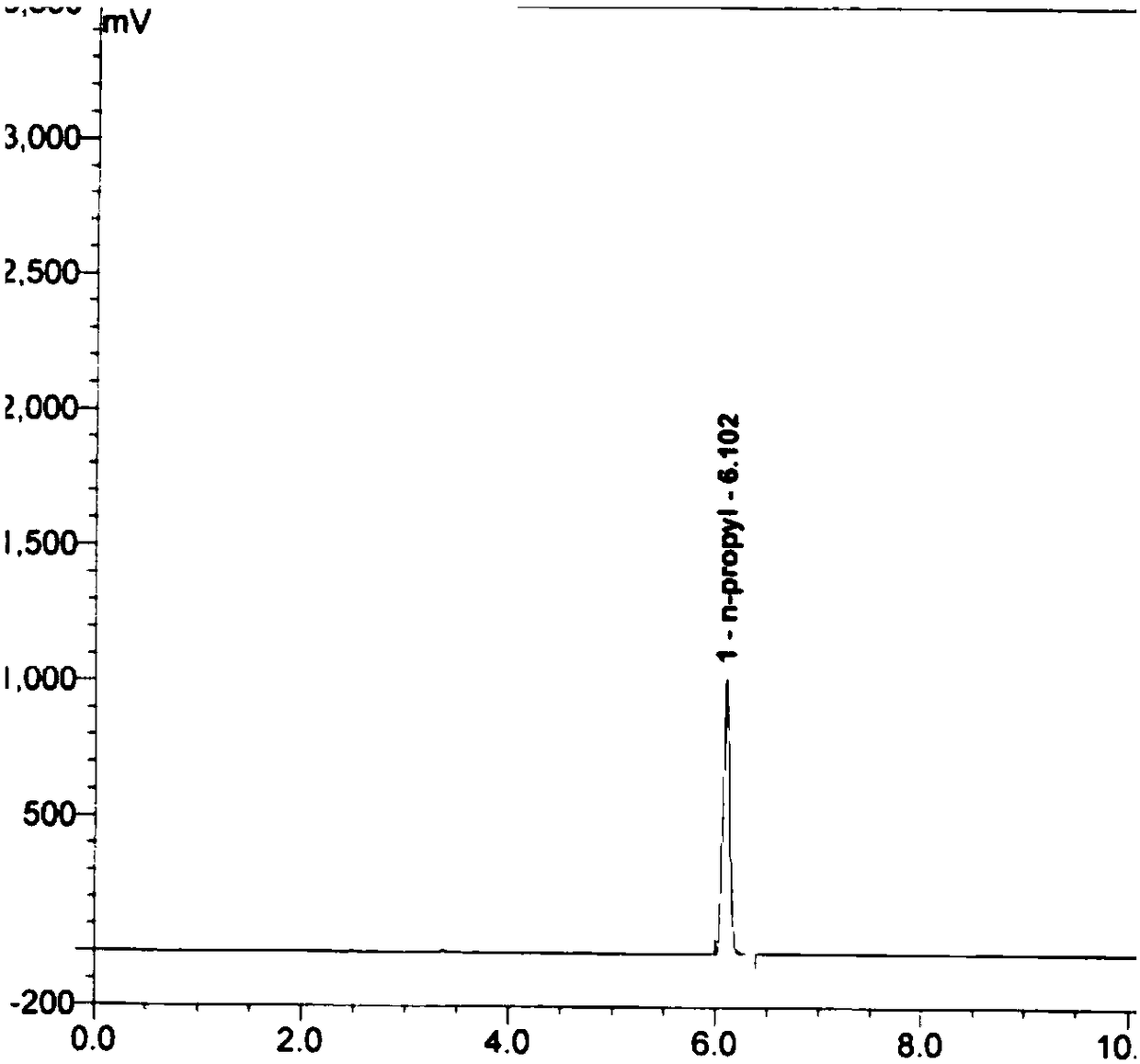
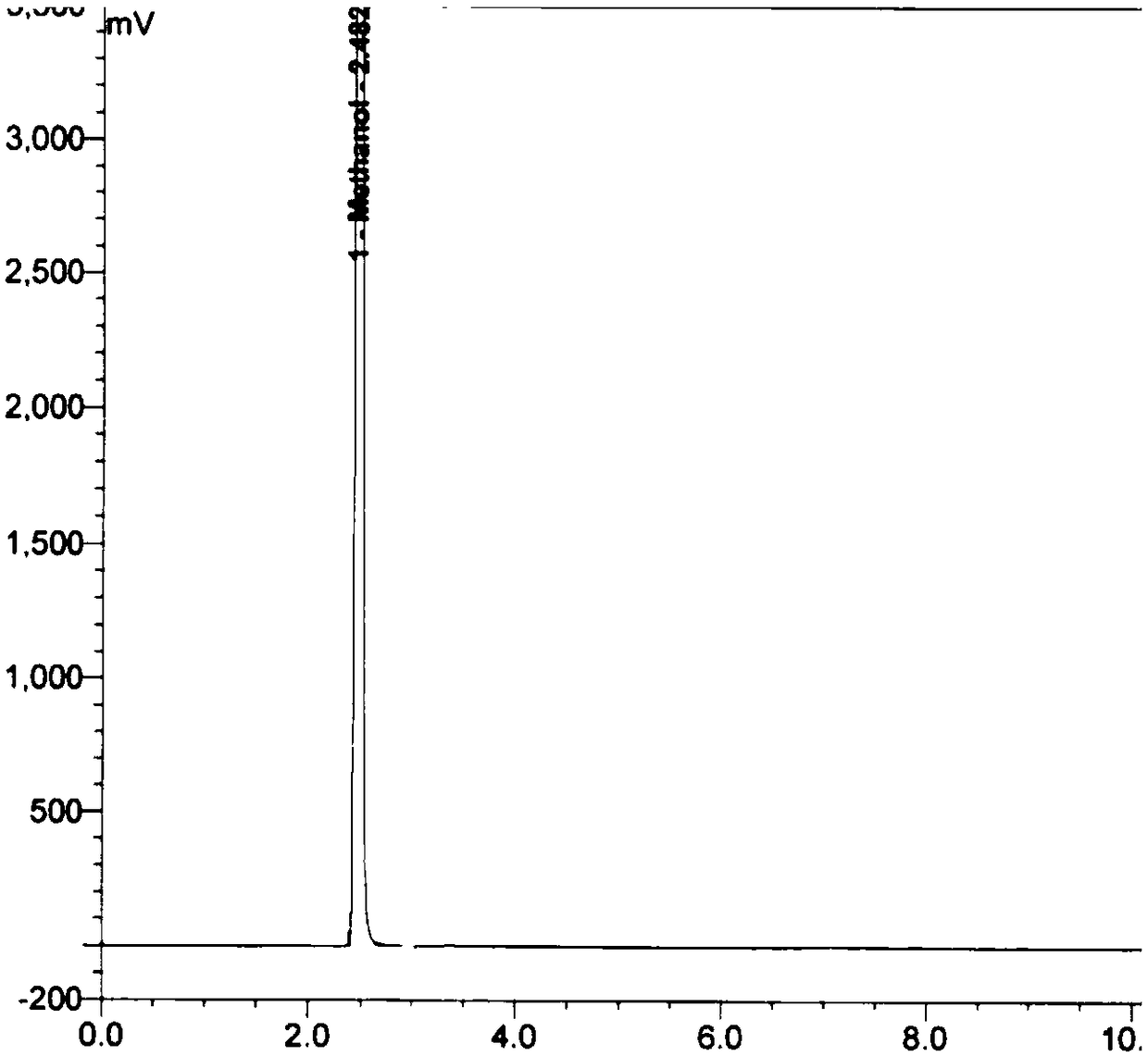
[0118] Get first 5 parts of mixed reference substance parallel samples in embodiment 1, each parallel sample injection needle carries out gas phase detection, record chromatogram, chromatogram is as follows Figure 9-13 shown. The data reflected in the chromatogram were sorted out and analyzed, and the analysis results of the system suitability test results are shown in Table 1.
[0119] Table 2 Results of solvent residual system suitability test (headspace equilibrium temperature 80°C)
[0120]
[0121]
[0122] As can be seen from the test results, each analyte is well separated under the gas chromatographic conditions established by the technical scheme of the present invention, the column efficiency meets the requirements, the RSD value of the peak area ratio repeatability of each analyte is less than 5.0%, and the method system applicability meets the requirements.
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com