A kind of coumarin derivative docopa and its preparation method and application
A technology of coumarin derivatives and coumarin, applied in chemical instruments and methods, fluorescence/phosphorescence, instruments, etc., can solve problems such as the limitation of fluorescent probes, the research on limiting the effects of physiology and pathology, etc., and achieve broad application Prospect, excellent sensitivity and selectivity, simple to synthesize effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Preparation of DOCOPA:
[0032] 1) Synthesis of compound I:
[0033]
[0034] Dissolve 3-acetyl-7-N,N-diethylaminocoumarin (6mmol, 1.55g) and p-hydroxybenzaldehyde (6mmol, 0.732g) in 30mL of acetonitrile, add 3-5 drops of piperidine, Reflux for 11 hours until the reaction is complete; the system is cooled to room temperature, filtered with suction, washed with acetonitrile, and the filter cake is dried to obtain compound I.
[0035] 2) Synthesis of compound DOCOPA:
[0036]
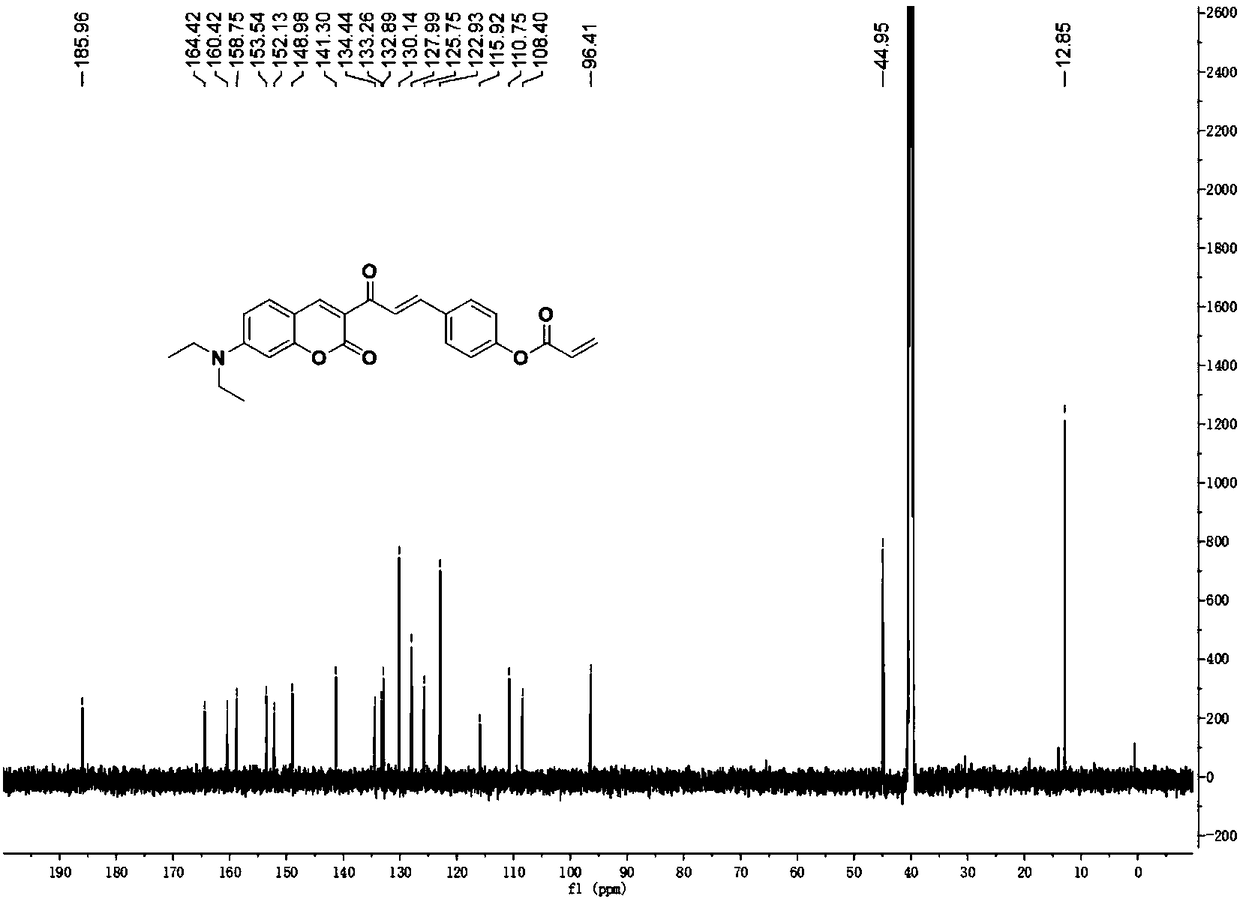
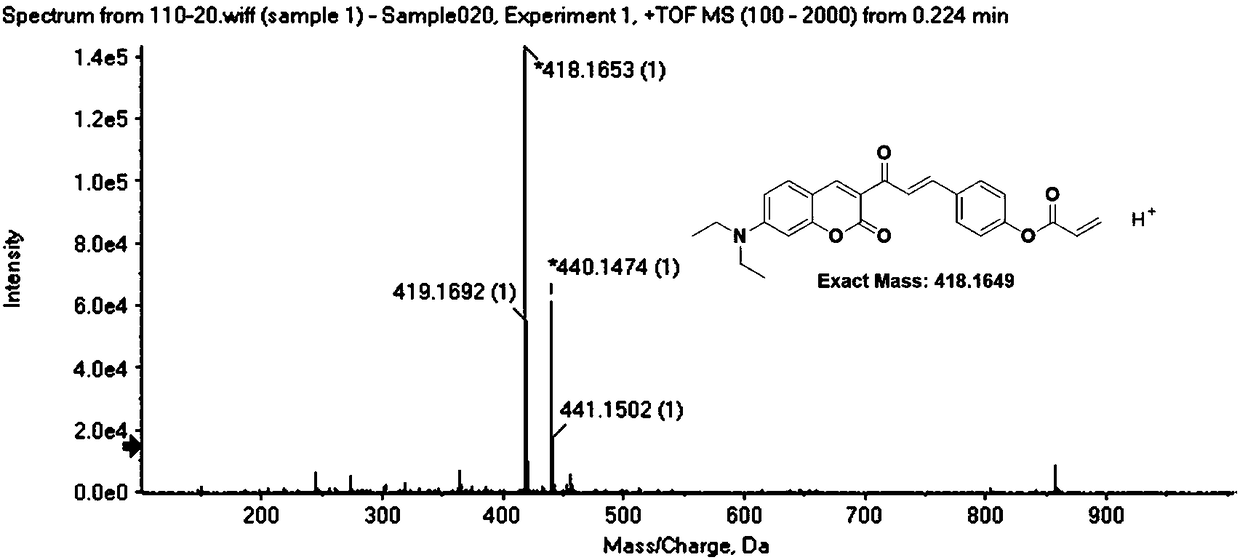
[0037] Dissolve compound I (1mmol, 0.36g) and triethylamine (1mmol) in dichloromethane, gradually add acryloyl chloride (1.4mmol) dropwise in an ice bath, stir at room temperature for 20 hours after the dropwise addition, until the reaction is complete; After being evaporated to dryness under pressure, the target compound DOCOPA was obtained by column chromatography (ethyl acetate:petroleum ether=1:3 by volume).
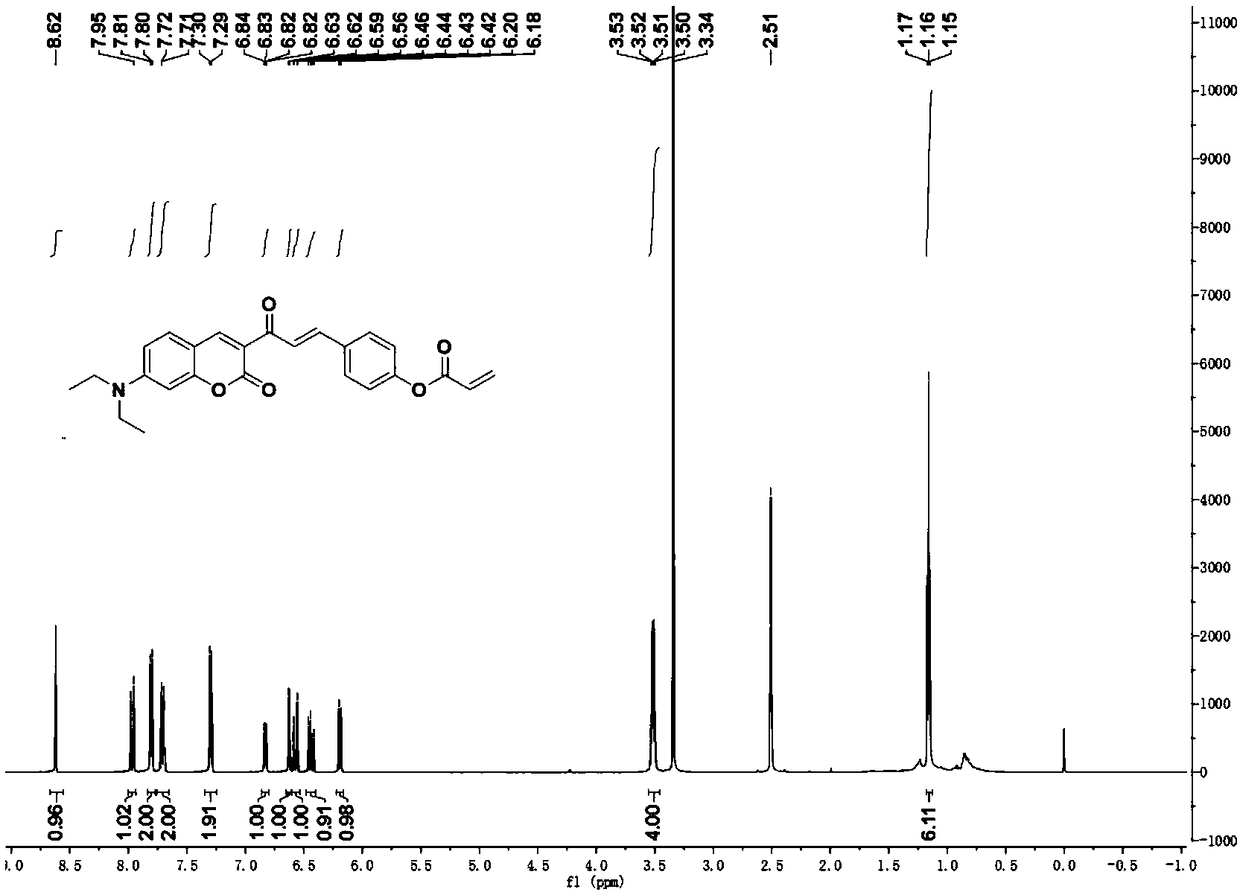
[0038] 1 H NMR (600MHz, DMSO-d 6 ):δ8.62(s,1H),7.96(d,J=15.8Hz,1H),7.80(d,...
Embodiment 2
[0040] Prepare HEPES buffer solution with pH=7.8 and concentration of 10mM, prepare 2mM DOCOPA in DMSO solution, prepare 20mM Cys aqueous solution; take 2mL of HEPES / DMSO (v / v, 1:1, pH 7.8) solution, 30μL DOCOPA in DMSO The solution was added to a fluorescence cuvette, and 30 μL Cys aqueous solution was added, and detected on a fluorescence spectrophotometer (447nm excitation) over time. Within 0-12min, the fluorescence intensity at 499nm gradually increased; after 12min, the fluorescence intensity at 499nm gradually decreased, and the fluorescence intensity at 554nm gradually increased. See the fluorescence emission diagram Figure 4 and Figure 5 .
Embodiment 3
[0042] Prepare HEPES buffer solution with pH=7.8 and concentration of 10mM, prepare 2mM DOCOPA in DMSO solution, prepare 20mM Hcy aqueous solution; take 2mL of HEPES / DMSO (v / v, 1:1, pH 7.8) solution, 30μL DOCOPA in DMSO The solution was added to a fluorescence cuvette, and 30 μL of Hcy aqueous solution was added, and detected on a fluorescence spectrophotometer (excitation at 447 nm) over time. The fluorescence intensity at 499nm increases gradually, and the fluorescence emission diagram is shown in Image 6 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com