Cysteine detection method
A cysteine and solution technology, used in material excitation analysis, fluorescence/phosphorescence, etc., can solve the problems of mutual interference and indistinguishable detection, and achieve the effects of low detection cost, good selectivity and fast detection speed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Using this method to differentiate cysteine and homocysteine
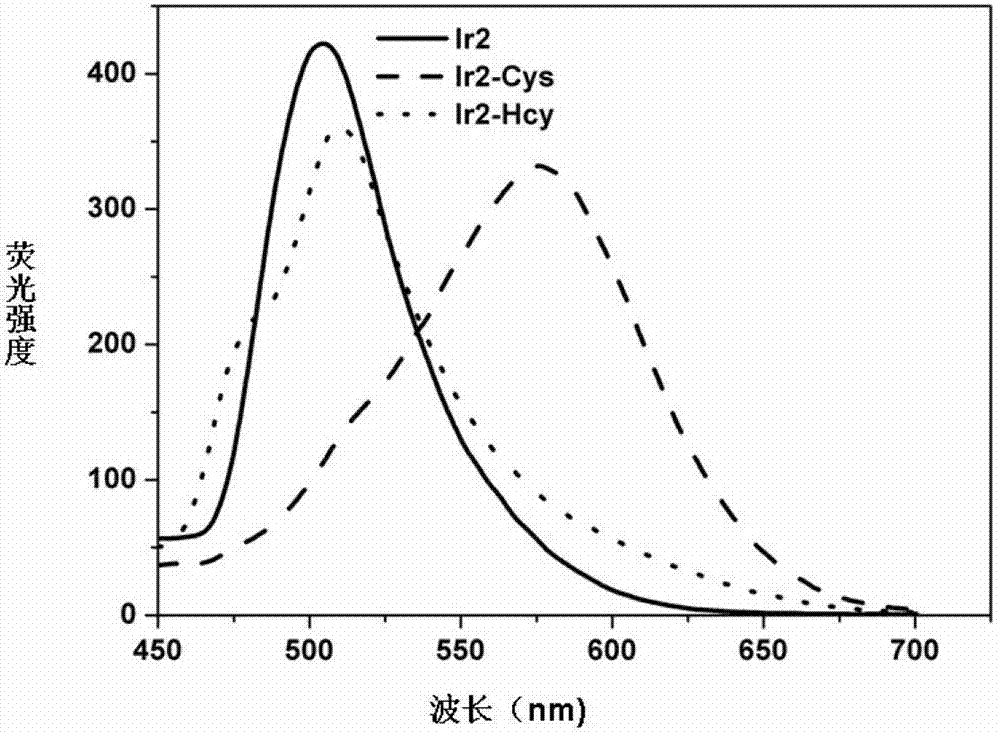
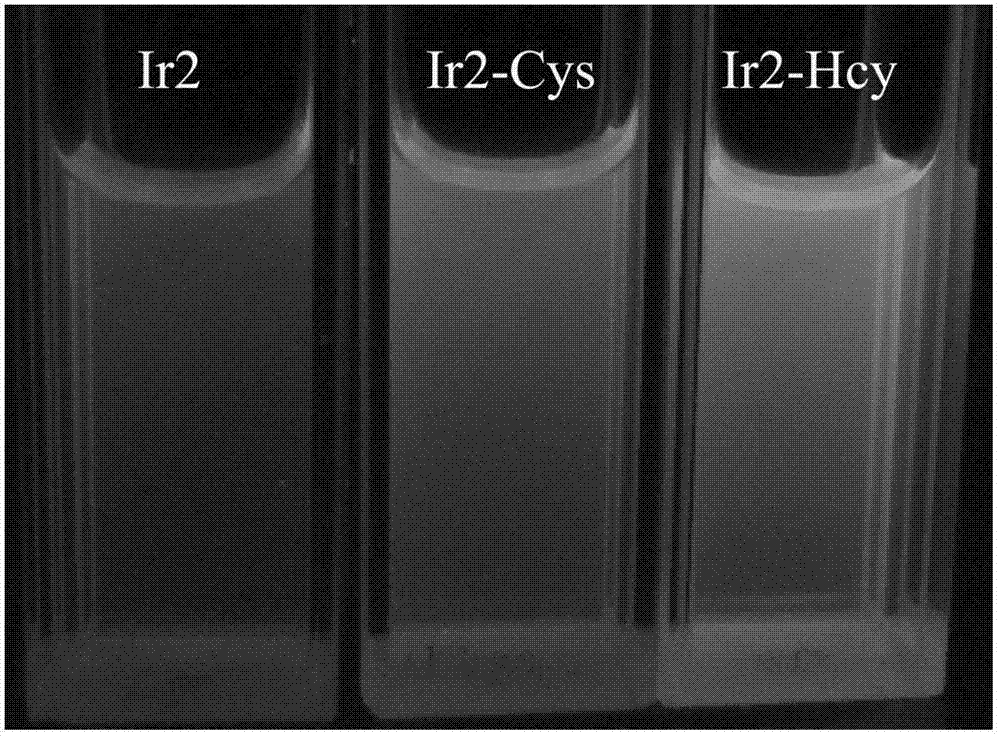
[0023] Prepare 2*10 with DMF -3 M's Ir 2 (ppy) 4 Cl 2 Solution, and prepare HEPES (0.2M) buffer solution with pH 7.0 with triple distilled water; respectively take 1750 μl DMF and 200 μl HEPES buffer solution and add them to the two fluorescence pools, and use a micro-sampler to absorb the above-mentioned Ir 2 (ppy) 4 Cl 2 Add 50 μl of the solution to a fluorescent dish and detect it on a fluorescent instrument. There is emission at 505 nm; take 4×10 -3 100 μl of cysteine and homocysteine solutions were gradually added to the above two fluorescent pools with a micro-injector, placed at room temperature for 20 minutes and then detected on a fluorescence instrument. The emission peak of the system added with cysteine was determined by 505nm red shifted to 576nm ( Figure 1A ). Irradiated with 365nm ultraviolet light from a portable ultraviolet lamp, the color of the emitted light chan...
Embodiment 2
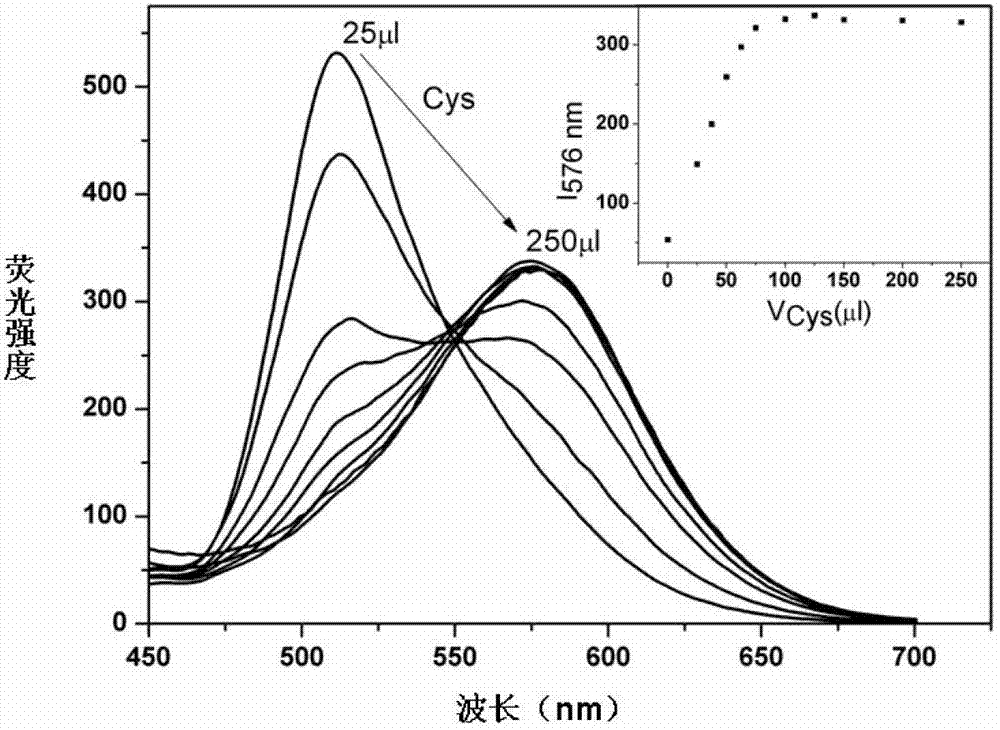
[0025] Prepare 2×10 in DMF -3 M's Ir 2 (ppy) 4 Cl 2 solution, and prepare HEPES (0.2M) buffer solution with pH 7.0 with triple distilled water; take 1750 μl DMF and 200 μl HEPES buffer solution and add them to the fluorescent cell, and use a micro-sampler to absorb the above-mentioned Ir 2 (ppy) 4 Cl 2 Add 50 μl of the solution to the fluorescence dish and detect it on the fluorescence instrument. There is emission at 505 nm; take the cysteine solution and gradually add it to the above-mentioned fluorescence pool with a micro-injector, and detect it on the fluorescence instrument while adding the sample. The emission peak of the gradually adding cystine system is red-shifted from 505nm to 576nm ( figure 2 ).
Embodiment 3
[0027] Prepare 2*10 with DMF -3 M's Ir 2 (ppy) 4 Cl 2 solution, and prepare HEPES (0.2M) buffer solution with pH 7.0 with triple distilled water; take 900 μl DMF and 500 μl HEPES buffer solution and add them to the fluorescent cell, and use a micro-sampler to absorb the above-mentioned Ir 2 (ppy) 4 Cl 2 Add 50 μl of the solution to the fluorescence dish and detect it on the fluorescence instrument. There is emission at 505 nm; take the cysteine solution and gradually add it to the above-mentioned fluorescence pool with a micro-injector, and detect it on the fluorescence instrument while adding the sample. The emission peak of the gradually added system of cystine shifted from 505nm to 570nm ( image 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com