A kind of coumarin derivative ss-590 and its preparation method and application
A technology of coumarin derivative, SS-590, which is applied in the field of analytical chemistry, can solve the problem that albumin cannot be used for quantitative analysis and labeling, and achieves the effects of easy large-scale production, low cost and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
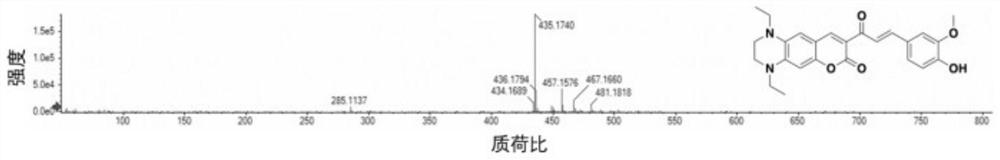
[0024] Preparation and characterization of SS-590:
[0025]
[0026] 8-Acetyl-1,4-diethyl-1,2,3,4-tetrahydro-7H-pyrano[2,3-g]quinoxalin-7-one (0.5 mmol, 0.15 g ), vanillin (0.6mmol, 0.09g) were dissolved in 20mL of ethanol, 3 to 5 drops of piperidine were added, and refluxed for 70h until the reaction was complete; the system was evaporated to dryness under reduced pressure to obtain a crude product, which was then separated by column chromatography (eluent according to Volume ratio of ethyl acetate: petroleum ether=1:1) to obtain fluorescent probe SS-590.
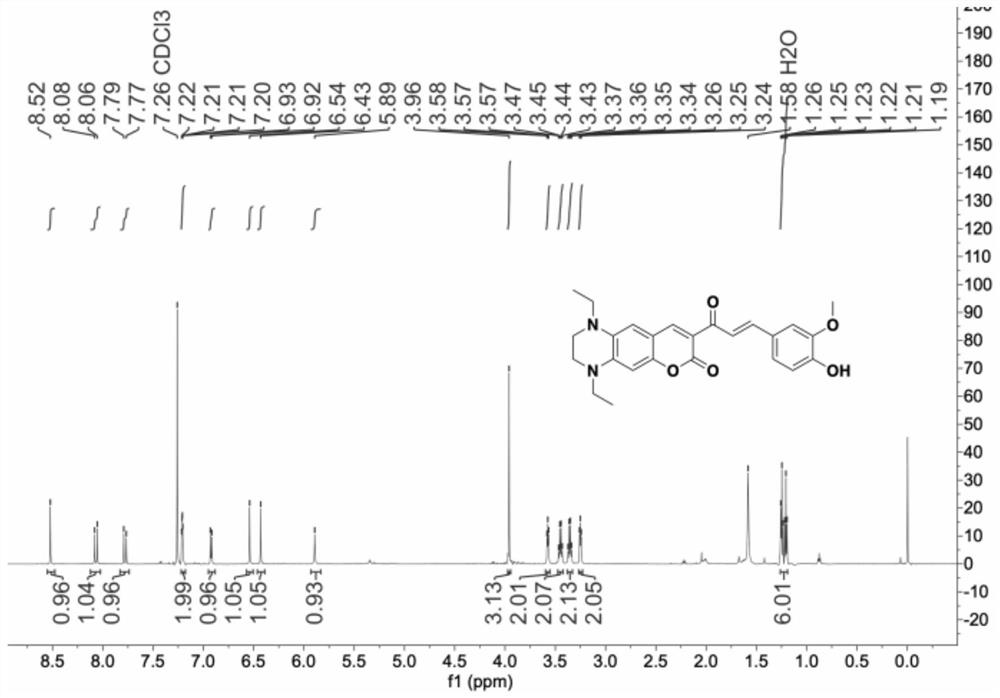
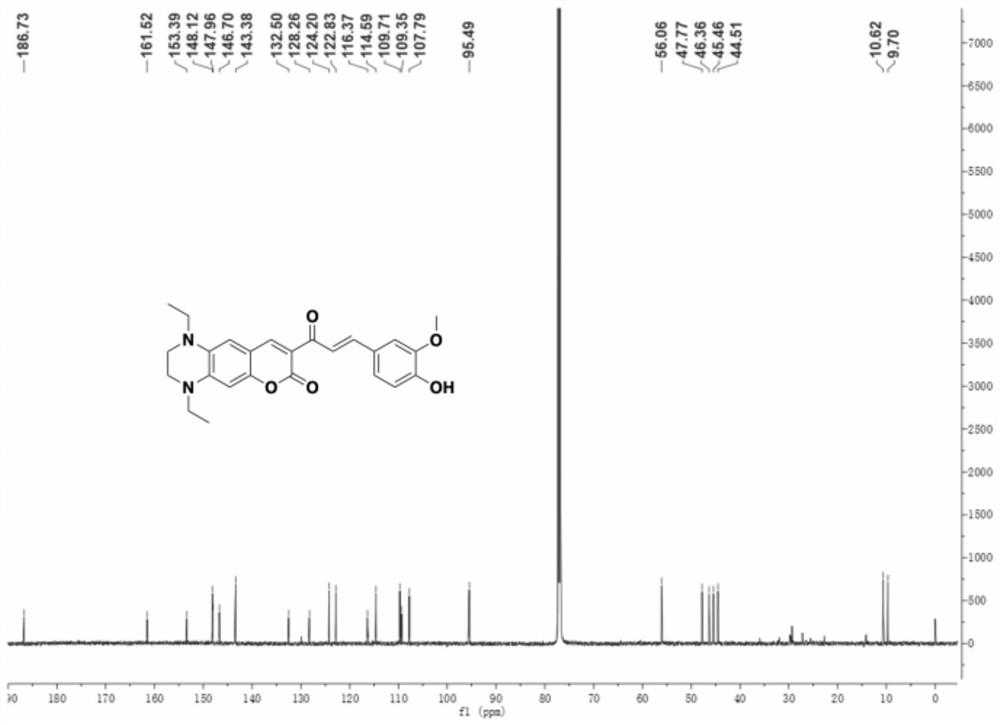
[0027] 1 H NMR (600MHz, Chloroform-d) δ8.52(s, 1H), 8.07(d, J=15.6Hz, 1H), 7.78(d, J=15.6Hz, 1H), 7.23–7.18(m, 2H) ,6.92(d,J=8.6Hz,1H),6.54(s,1H),6.43(s,1H),5.89(s,1H),3.96(s,3H),3.59–3.55(m,2H), 3.45(q,J=7.1Hz,2H),3.36(q,J=7.1Hz,2H),3.27–3.23(m,2H),1.25(t,J=7.1Hz,3H),1.21(t,J =7.1Hz,3H)( figure 1 ). 13 C NMR (151MHz, CDCl 3 )δ186.73,161.52,153.39,148.12,147.96,146.70,143.38,132.50,128.26,124.20,122.83,116.37,114...
Embodiment 2
[0029] Prepare PBS solution, prepare 2mM SS-590 in DMSO, prepare 20mg / mL human serum albumin in water; take 1950μL of PBS, 5μL of SS-590 in DMSO solution and add it to a fluorescence cuvette, add 50μL of human serum albumin The aqueous solution of protein was mixed evenly and detected on a spectrofluorometer (excitation at 505 nm) after 10 min. The fluorescence intensity at 590nm gradually increases, and the fluorescence emission diagram is shown in Figure 4 .
Embodiment 3
[0031] Prepare PBS solution, prepare 2mM SS-590 DMSO solution, prepare 20mg / mL human serum albumin aqueous solution, prepare 20mg / mL bovine serum albumin aqueous solution, prepare 10mg / mL mouse serum albumin aqueous solution, prepare 8mM Glu, respectively, Ile, Lys, Leu, Met, Phe, Ser, Hyp, Gln, Pro, Tyr, Cystine, Arg, Thr, Asp, Na 2 SO 3 , Na 2 S, H 2 O 2 , NaClO, 4mM Hcy, 40mM Cys and 80mM GSH aqueous solution; in 27 fluorescence cuvettes, take 1950μL of PBS and 5μL of DMSO solution of SS-590 into the fluorescence cuvette respectively, add 50μL of PBS, Glu , Ile, Lys, Leu, Met, Phe, Ser, Hyp, Gln, Pro, Tyr, Cystine, Arg, Thr, Asp, Na 2 SO 3 , Na 2 S, Cys, Hcy, GSH, H 2 O 2 , NaClO, Gly, mouse serum albumin, bovine serum albumin, and human serum albumin solutions were mixed, and the fluorescence intensity was detected on a fluorescence spectrophotometer after 10 min (excitation at 505 nm). Only the human serum albumin added group showed a significant increase in fluo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com