Separation and detection method of acetylcysteine enantiomer
A technology of acetylcysteine and enantiomers, applied in the field of separation and detection of acetylcysteine, can solve the problems of unfavorable popularization, complex operation, low sensitivity, etc. Mild, short retention time effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
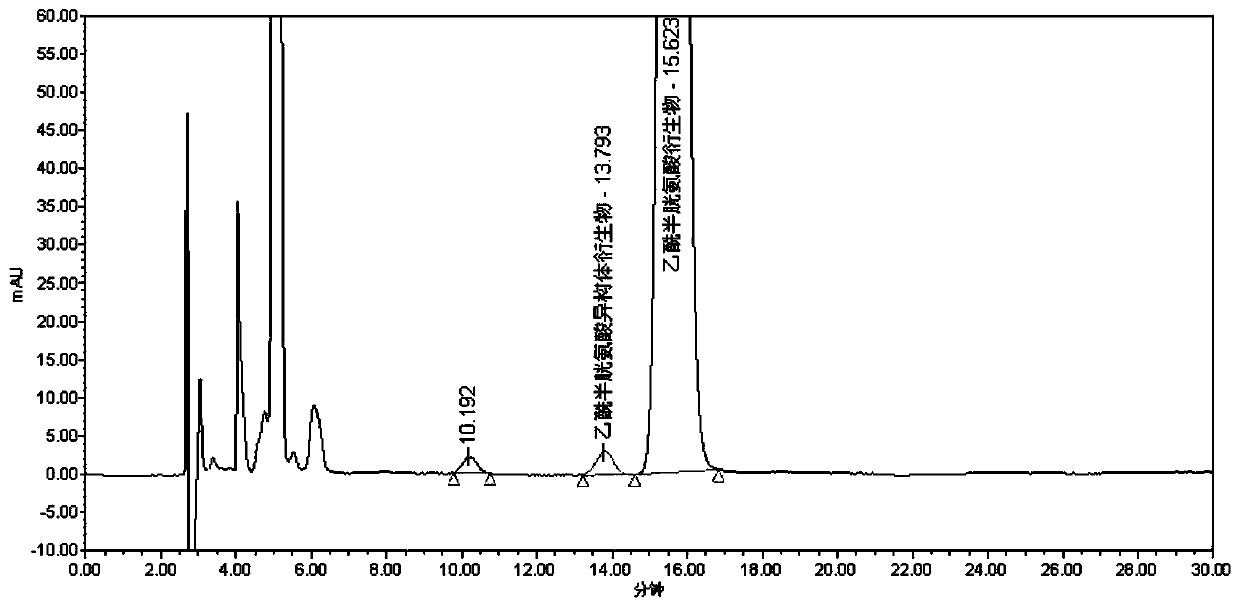
[0075] Sample to be tested 1: Accurately measure 1ml of acetylcysteine solution for inhalation, the pH of the solution is neutral, put it in a 100ml measuring bottle, dilute with methanol to the mark, shake well, take 5ml precisely, put it in a 10ml measuring bottle ,stand-by;
[0076] Take another 25 mg of acetylcysteine enantiomer reference substance, accurately weigh it, put it in a 25ml measuring bottle, add 3ml of 0.05mol / L sodium hydroxide methanol solution, dilute to the mark with methanol, shake well, and use it as a reference substance Mother liquor: Accurately measure 1ml of the mother liquor of the reference substance, put it in a 20ml measuring bottle, dilute to the mark with methanol, shake well, accurately measure 1ml, add it to the above-mentioned sample solution to be used, and then precisely add the derivative reagent (R)-( +)-a-methylbenzyl isocyanate 10μl, plugged tightly, shake well, put in 55°C water bath for 20min, let cool, dilute to the mark with me...
Embodiment 2
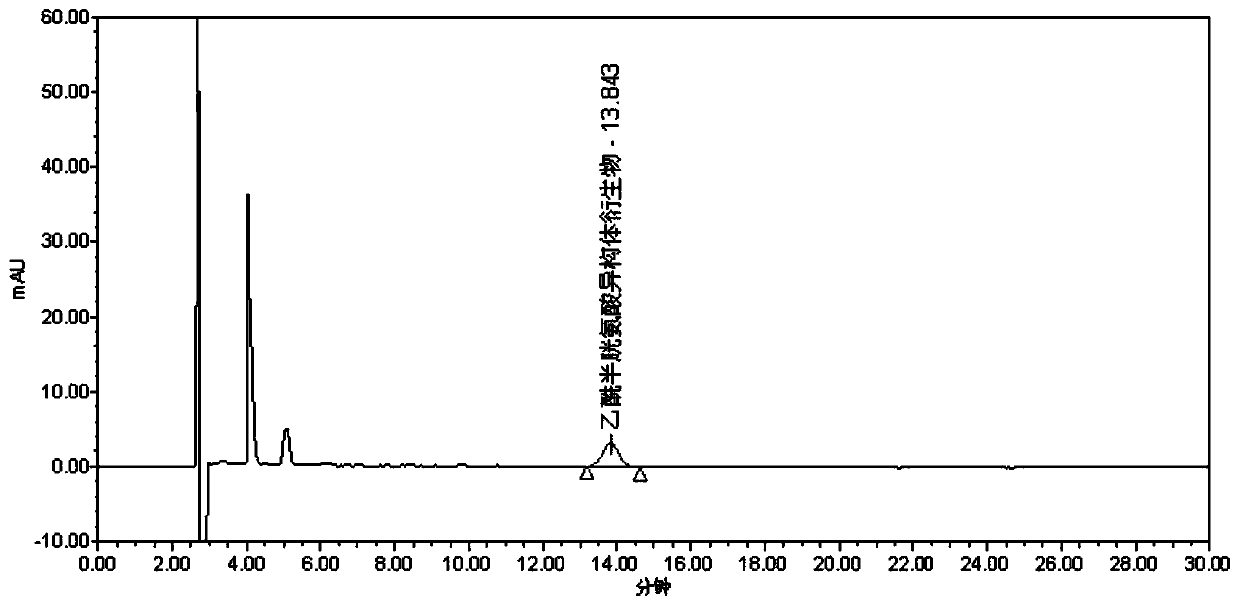
[0081] Sample to be tested 1: Accurately measure 1ml of acetylcysteine solution for inhalation. The pH of the solution is neutral. in a bottle, ready to use;
[0082] Take another 25 mg of acetylcysteine isomer reference substance, accurately weigh it, put it in a 25 ml measuring bottle, add 3 ml of 0.05 mol / L sodium hydroxide isopropanol solution, dilute to the mark with isopropanol, shake well, and use as Reference substance mother liquor: accurately measure 1ml of the reference substance mother liquor, put it in a 20ml measuring bottle, dilute to the mark with isopropanol, shake up, accurately measure 1ml, add it to the above-mentioned sample solution to be used, and then precisely add the derivative reagent ( S)-(-)-a-methylbenzyl isocyanate 10μl, plugged tightly, shake well, put in 50°C water bath for 18min, let cool, dilute to the mark with isopropanol, shake well, as a mixed solution;
[0083] Accurately measure 10 μl each of the reference substance solution and th...
Embodiment 3
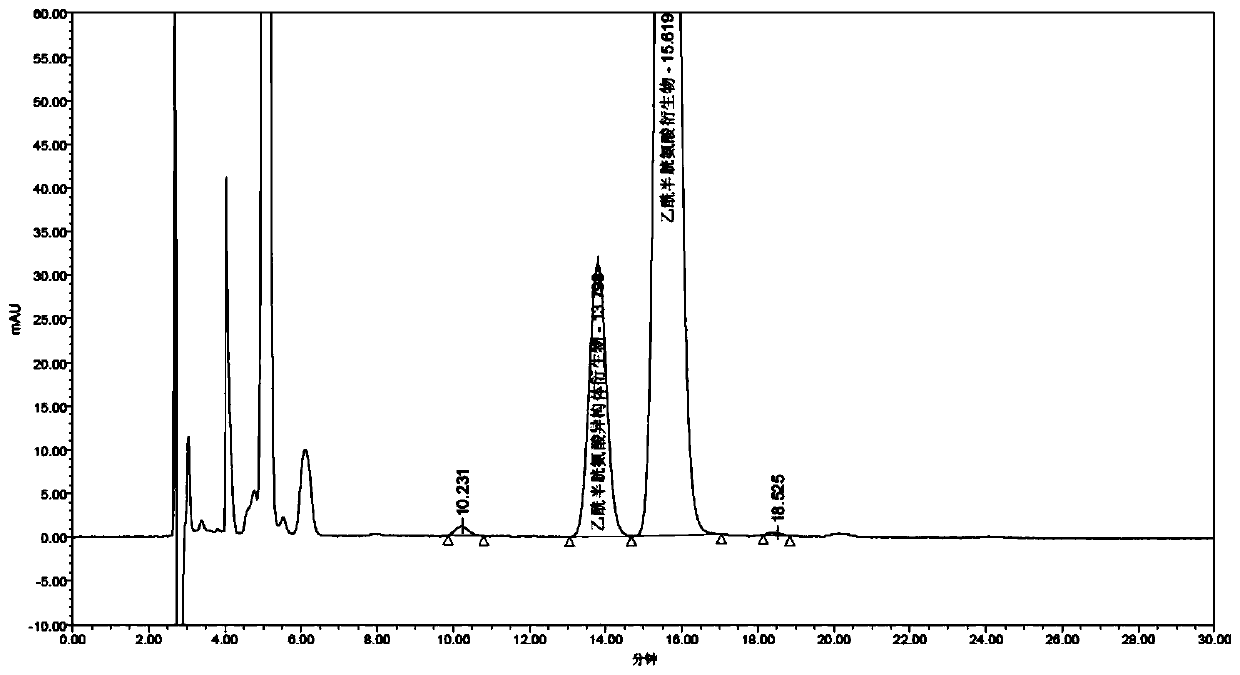
[0086] Sample to be tested 2: Accurately measure 1ml of acetylcysteine solution for inhalation. The pH of the solution is neutral. in a bottle, ready to use;
[0087] Take another 25 mg of acetylcysteine isomer reference substance, accurately weigh it, put it in a 25 ml measuring bottle, add 3 ml of 0.05 mol / L sodium hydroxide isopropanol solution, dilute to the mark with isopropanol, shake well, and use as Reference substance mother liquor: accurately measure 1ml of the reference substance mother liquor, put it in a 20ml measuring bottle, dilute to the mark with isopropanol, shake up, accurately measure 1ml, add it to the above-mentioned sample solution to be used, and then precisely add the derivative reagent ( S)-(-)-a-methylbenzyl isocyanate 10μl, plugged tightly, shake well, put in 50°C water bath for 18min, let cool, dilute to the mark with isopropanol, shake well, as a mixed solution;
[0088] Accurately measure 10 μl each of the reference substance solution and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com