A fingerprint detecting method for a Shensu preparation
A detection method and fingerprint technology, applied in the field of detection of fingerprints of ginseng preparations, can solve the problems of inability to effectively control the production process and product quality, and cannot better ensure clinical efficacy, and achieve a simple and well-correlated pretreatment method Good reproducibility and reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
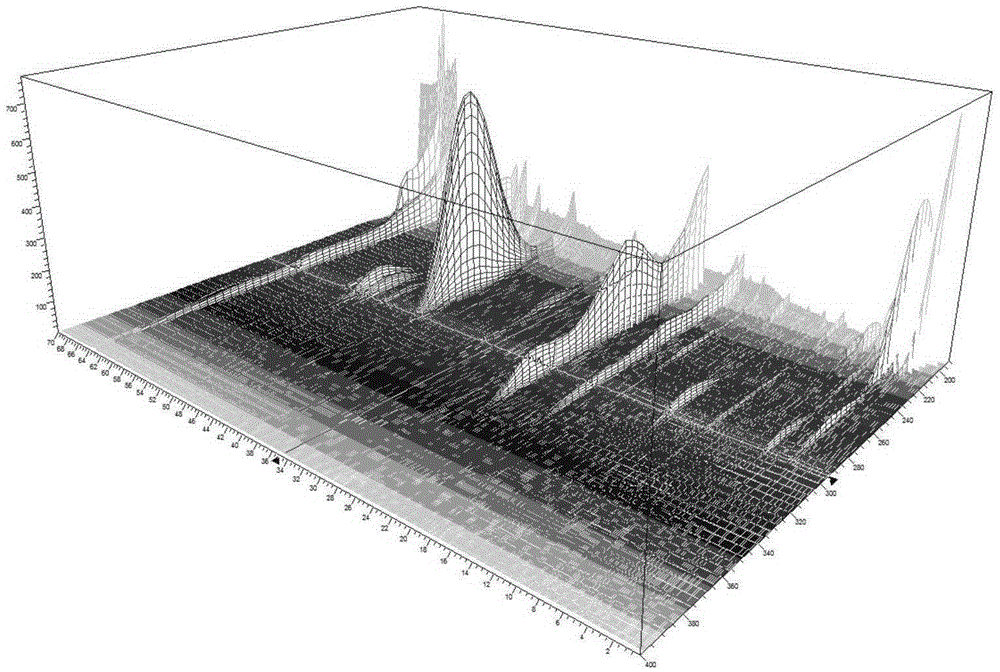
Image
Examples
Embodiment 1
[0085] The detection method of embodiment 1 ginseng preparation fingerprint chromatogram
[0086] The detection method of ginseng preparation comprises the following steps:
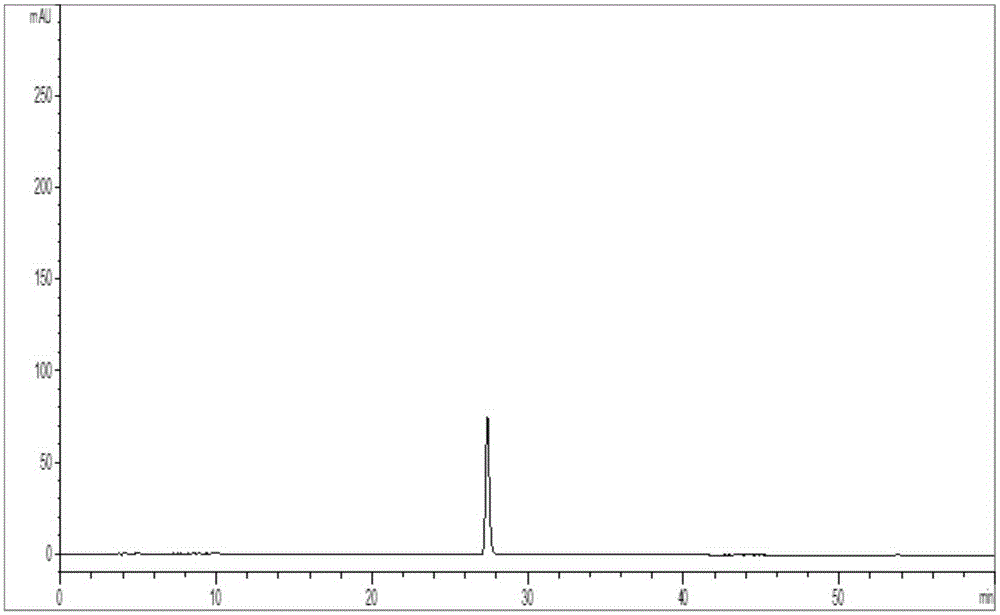
[0087] (a) Preparation of the reference substance solution: weigh the puerarin reference substance, place it in a volumetric flask, add 50% to 100% methanol to dissolve it and set the volume to the mark, shake well, and make the reference substance solution;
[0088] (b) Preparation of the test solution: take the ginseng preparation, add 0% to 50% methanol or 0% to 50% ethanol 25 to 125mL, extract by conventional methods, filter with a microporous membrane, and take the subsequent filtrate, Get the test solution;
[0089] (c) Chromatographic conditions: the chromatographic column is C 18 Reverse-phase chromatographic column; using gradient elution, the mobile phase is a gradient eluent composed of mobile phase A and mobile phase B, mobile phase A is acetonitrile, mobile phase B is 0.05-0.1% phosphoric a...
Embodiment 2
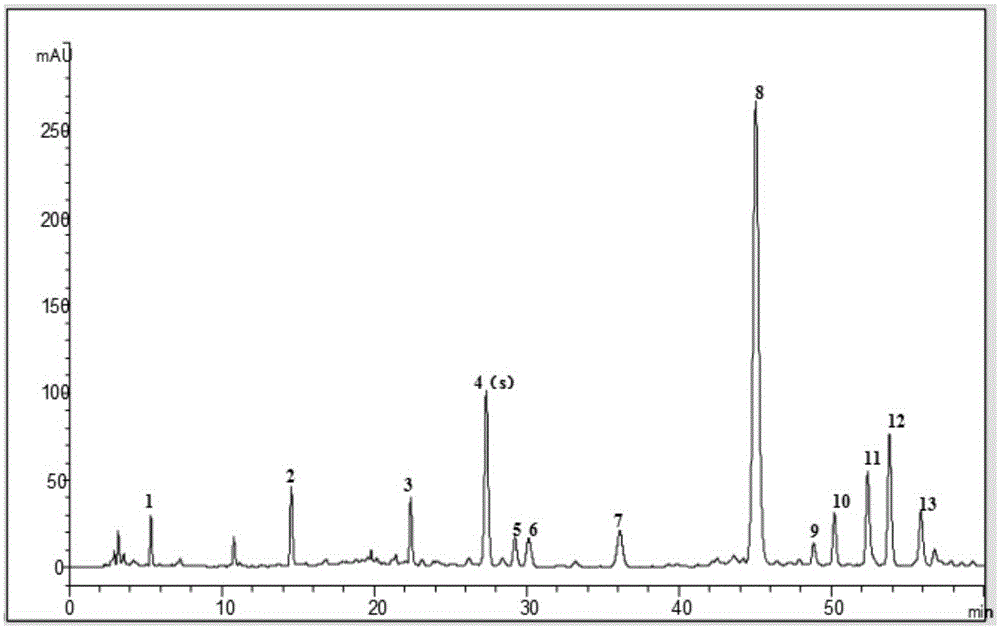
[0093] Example 2 Establishment of Fingerprint Spectrum of Shensu Preparation Oral Liquid
[0094] (1) Instruments and reagents
[0095]Agilent 1260 HPLC, including online vacuum degasser (G-1311C), binary pump (G-1311C), standard autosampler (G-1329B), intelligent column oven (G-1316A) , DAD detector (G-1314B), Agilent1260Infinity chromatographic workstation (Agilent Technologies Co., Ltd.); SB-5200D ultrasonic cleaner (Ningbo Xinzhi Biotechnology Co., Ltd.); FAZ004B analytical balance (Shanghai Youke); BT125D electronic balance (Sartorius Scientific Instruments (Beijing) Co., Ltd.).
[0096] (2) Chromatographic conditions
[0097] The column is Thermo Syncronis-C 18 (5μm, 4.6×250mm) reverse-phase chromatographic column; column temperature is 25°C; detection wavelength is 285nm; mobile phase A is acetonitrile, mobile phase B is 0.08% phosphoric acid solution, the total flow rate is 1.0mL / min; analysis time is 60 minutes ; The elution gradient is shown in Table 2.
[0098]...
Embodiment 3
[0120] Example 3 Establishment of Fingerprint of Shensu Preparation Intermediates
[0121] (1) Instruments and reagents
[0122] Agilent 1260 HPLC, including online vacuum degasser (G-1311C), binary pump (G-1311C), standard autosampler (G-1329B), intelligent column oven (G-1316A) , DAD detector (G-1314B), Agilent1260Infinity chromatographic workstation (Agilent Technologies Co., Ltd.); SB-5200D ultrasonic cleaner (Ningbo Xinzhi Biotechnology Co., Ltd.); FAZ004B analytical balance (Shanghai Youke); BT125D electronic balance (Sartorius Scientific Instruments (Beijing) Co., Ltd.).
[0123] (2) Chromatographic conditions
[0124] The column is Thermo Syncronis-C 18 (5μm, 4.6×250mm) reverse-phase chromatographic column; column temperature is 30°C; detection wavelength is 283nm; mobile phase A is acetonitrile, mobile phase B is 0.05% phosphoric acid solution, the total flow rate is 1.0mL / min; analysis time is 60 minutes ; The elution gradient is shown in Table 4.
[0125] Table...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com