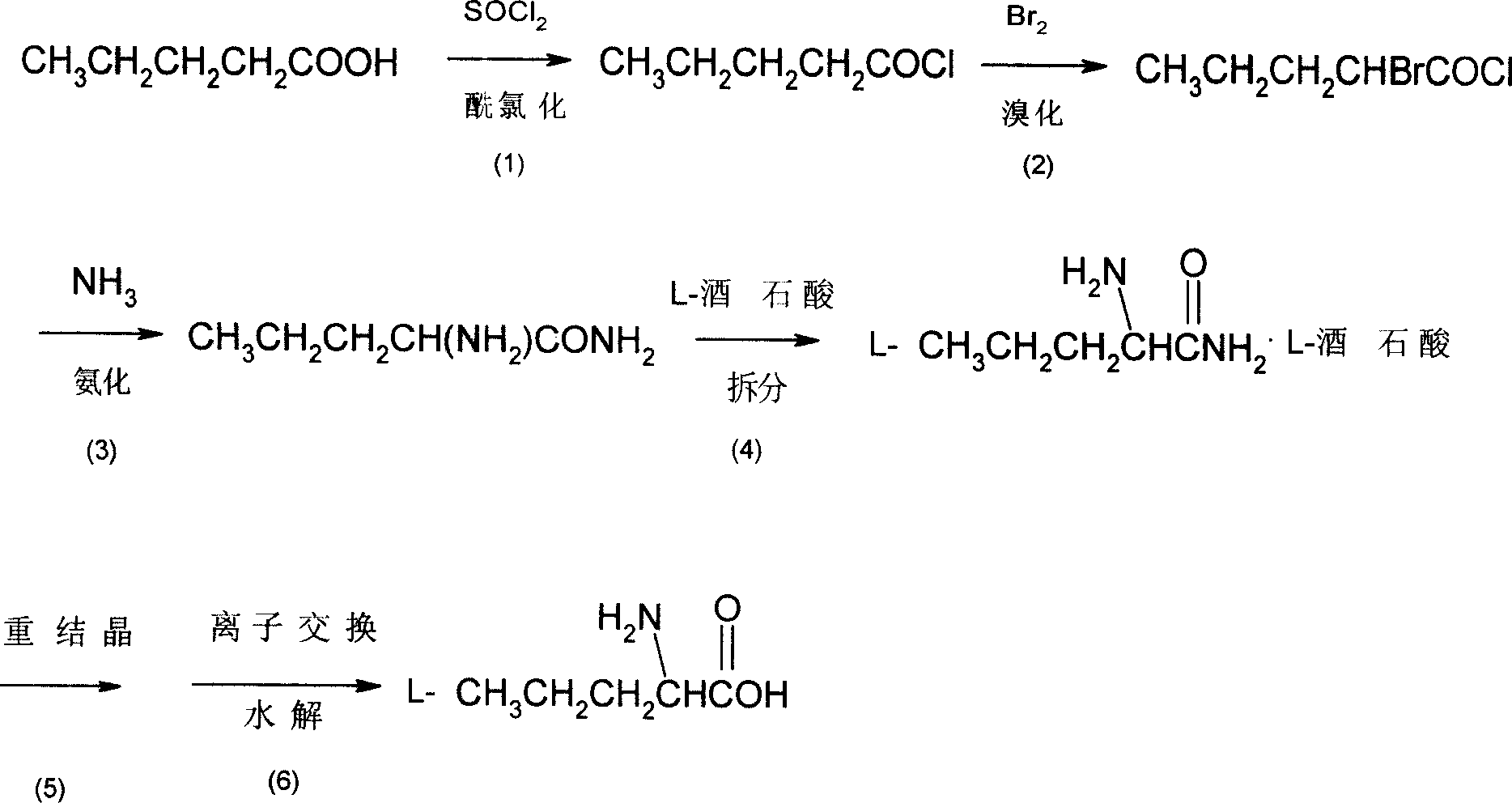Method for synthesis of L-norvaline
A synthesis method and technology of norvaline, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as difficulty in meeting production needs, achieve low cost, large output, and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
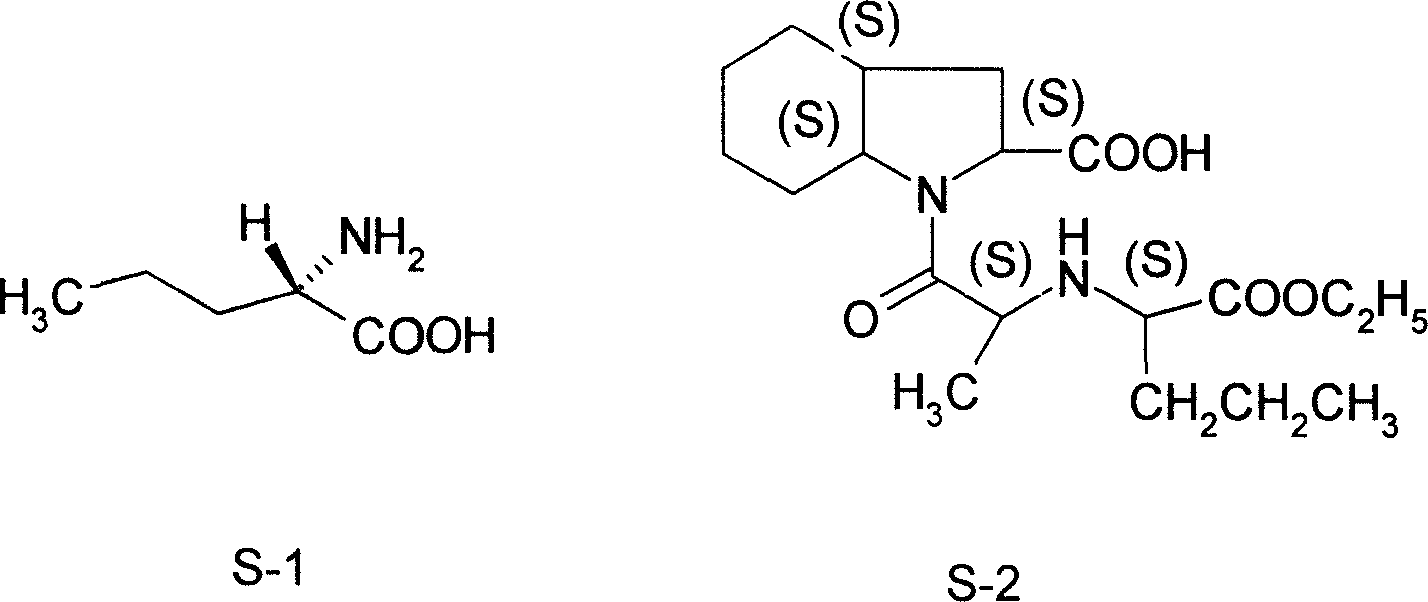
[0020] Embodiment 1: A kind of synthetic method of L-norvaline, take n-valeric acid as main starting material, make through following steps successively:
[0021] (1) Acyl chloride, i.e. the preparation of n-pentanoyl chloride:
[0022] In the flask equipped with stirring, reflux condenser, dropping funnel, calcium chloride drying tube and thermometer, add n-valeric acid (36.7g, 0.36mol) and thionyl chloride (123.9g, 1.04mol) successively, oil Bath heating, reflux at 78 ° C for 4 hours, until no hydrogen chloride gas is released.
[0023] (2) bromination, preparation of α-bromovaleryl chloride:
[0024] Keep the liquid obtained in the previous step slightly boiling, and the reaction temperature is 80°C. Liquid bromine (60.5 g, 0.38 mol) dried with an equal volume of concentrated sulfuric acid is added dropwise to the flask, and the dropping process is completed within 5 hours. After the dropwise addition was completed, heating and stirring were stopped, and the mixture was a...
Embodiment 2
[0034] Embodiment 2: a kind of synthetic method of L-norvaline, take n-valeric acid as main starting material, make through following steps successively:
[0035] (1) Acyl chloride, i.e. the preparation of n-pentanoyl chloride:
[0036] In the flask equipped with stirring, reflux condenser, dropping funnel, calcium chloride drying tube and thermometer, add n-valeric acid (36.7g, 0.36mol) and thionyl chloride (42.9g, 0.36mol) successively, oil Bath heating, reflux at 50 ° C for 8 hours, until no hydrogen chloride gas is released.
[0037] (2) bromination, preparation of α-bromovaleryl chloride:
[0038] Keeping the temperature of the liquid obtained in the previous step at 50°C, liquid bromine (115.2 g, 0.72 mol) dried with an equal volume of concentrated sulfuric acid was added dropwise to the flask, and the dropping process was completed within 1 h. After the dropwise addition was completed, heating and stirring were stopped, and the mixture was allowed to stand overnight. ...
Embodiment 3
[0048] Embodiment 3: a kind of synthetic method of L-norvaline, take n-valeric acid as main starting material, make through following steps successively:
[0049] (1) Acyl chloride, i.e. the preparation of n-pentanoyl chloride:
[0050] In the flask equipped with stirring, reflux condenser, dropping funnel, calcium chloride drying tube and thermometer, add n-valeric acid (36.7g, 0.36mol) and thionyl chloride (214.4g, 1.80mol) successively, water bath React at 10°C for 1h.
[0051] (2) bromination, preparation of α-bromovaleryl chloride:
[0052] Keeping the temperature of the liquid obtained in the previous step at 65°C, liquid bromine (86.4 g, 0.54 mol) dried with an equal volume of concentrated sulfuric acid was added dropwise to the flask, and the dropping process was completed within 10 h. After the dropwise addition was completed, the stirring was stopped and left to stand overnight. The next day, rectification under reduced pressure collected 64.0 g of 88-90°C / 9mmHg f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com