Compounds
A compound and residue technology, applied in the field of novel polymyxin compounds, can solve problems such as reducing nephrotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
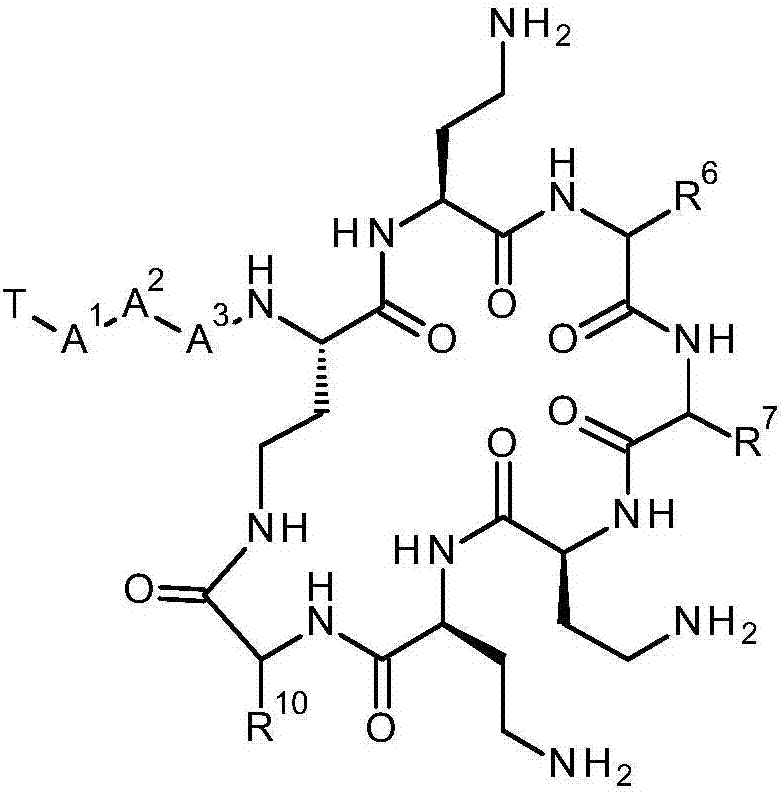
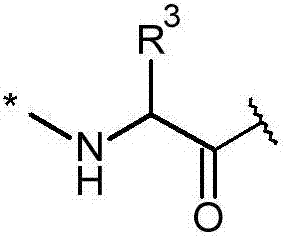
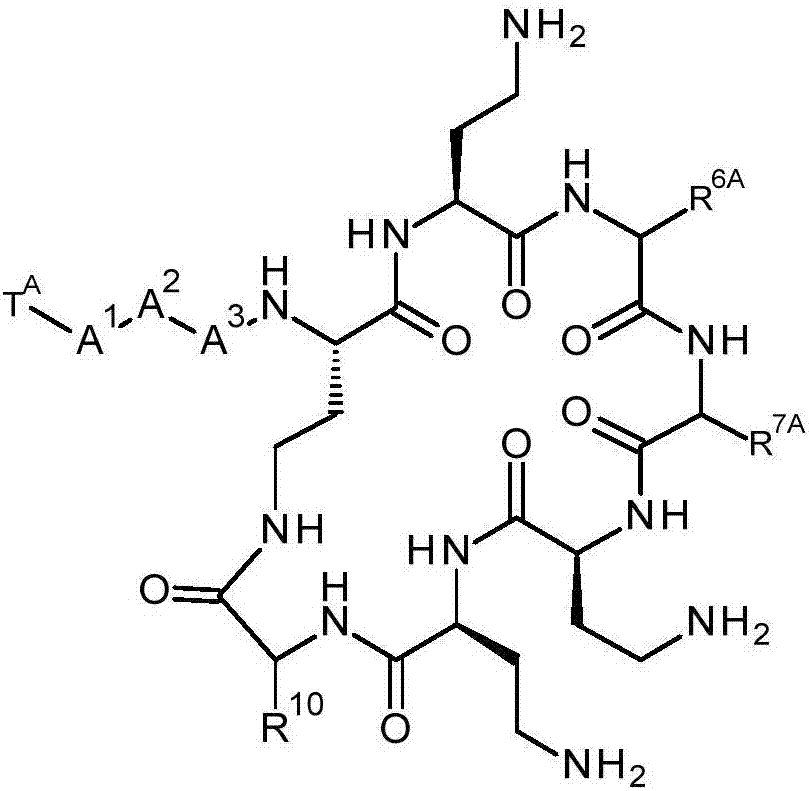
[0719] In one aspect of the present invention, there is provided a synthetic method comprising the steps of: digesting a halogenated polymyxin compound selected from the group consisting of halogenated decapeptides, halogenated nonapeptides and halogenated octapeptides, thereby producing halogenated heptapeptide polymyxins Bacteroid compounds. Digestion refers to the step of reducing the total number of amino acid residues within a polypeptide.
[0720] In one embodiment, a compound of formula (IVa) is digested to yield a compound of formula (IVb).
[0721] The compound of formula (IVa) is a halogenated decapeptide, halogenated nonapeptide or halogenated octapeptide. The compound of formula (IVa) is the compound of formula (IV), wherein-A 3 - is an amino acid residue. The compound of formula (IVa) is -R 6 or -R 7 Compounds containing haloaryl groups. After the cleavage reaction, the haloaryl group remains in the cleavage product.
[0722] The compound of formula (IVb) i...
Embodiment
[0919] The following examples are provided solely to illustrate the invention and are not intended to limit the scope of the invention described herein.
[0920] Nomenclature: Compounds are named based on the natural polymyxin core from which they are synthetically derived.
[0921]
[0922] Abbreviation Meaning
[0923] DCM dichloromethane
[0924] TFA trifluoroacetic acid
[0925] ND not determined
[0926] N / A not applicable
[0927] DMF N,N-Dimethylformamide
[0928] PMBH Polymyxin B Heptapeptide (3-10)
[0929] PMBD polymyxin B decapeptide
[0930] Pro Proline
[0931] Dap α,β-Diaminopropionic acid
[0932] Gly glycine
[0933] His Histidine
[0934] Phe Phenylalanine
[0935] DCHA dicyclohexylamine
[0936] X phos 2-Dicyclohexylphosphino-2′,4′,6′-triisopropylbiphenyl
[0937] NorLeu Norleucine
[0938] NorVal Norvaline
[0939] OctGly Octylglycine
[0940] Synthetic example
[0941] Preparation of comparative compounds C1 to C3.
[0942] Polymyxin B ...
Embodiment 24
[1007] Example 24: Polymyxin B[D-(4-cyano)Phe]-6
[1008] In anhydrous DMF (2ml) (Boc) 5 D-[(4-bromo)Phe]-6-polymyxin (100mg, 0.056mmol), zinc cyanide (45mg, 0.383mmol, 6.8mol equivalent) and 1,1'-bis(diphenylphosphino ) ferrocene (6 mg, 2 mol equiv) was degassed and then treated with tris(dibenzylideneacetone)dipalladium(0) (5 mg, 1 mol equiv). Seal the tube and heat to 100 °C for up to 3 days. The solvent was evaporated and the residue was partitioned between water and ethyl acetate. The organic phase was dried over anhydrous magnesium sulfate and evaporated. The residue was chromatographed on silica eluting with 0-10% in ethyl acetate (1% .880 ammonia in methanol), followed by further purification by preparative HPLC with 20%-95% acetonitrile in water (additional 1% TFA) elution. Fractions containing product were combined and evaporated to an oil. This oil was dissolved in TFA (2 mL) and DCM (8 mL) and stirred at room temperature for 6 hours. Evaporation of the solv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com