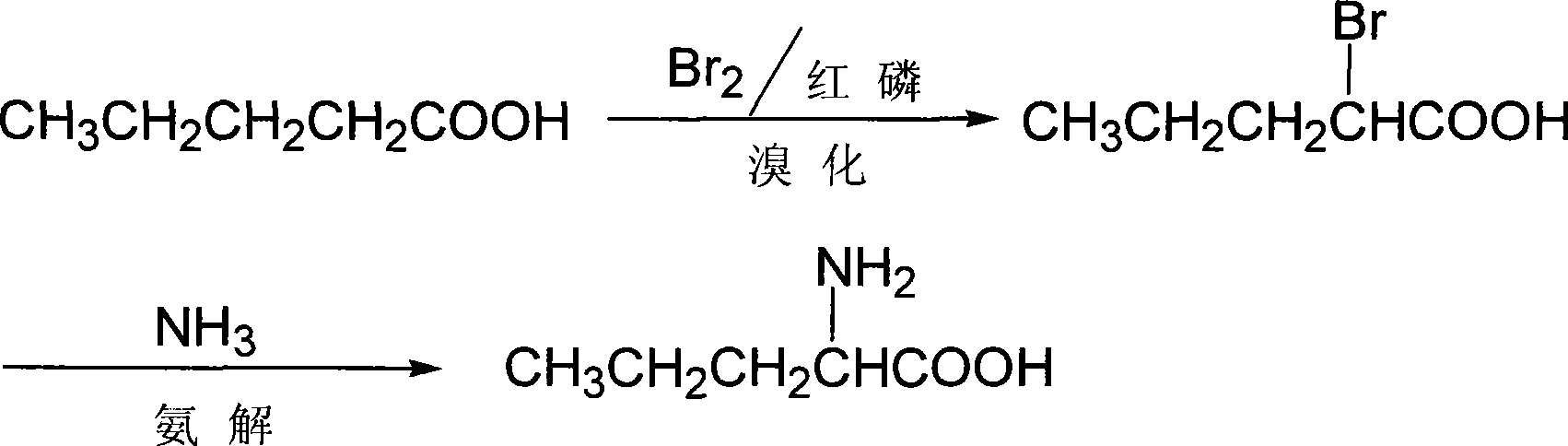
Synthesis of D,L-norvaline
A norvaline and synthesis method technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of low total yield, many reaction steps, and difficulty in obtaining acetone cyanohydrin, etc., and achieve The effect of abundant raw material sources, few synthesis steps, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] D, the synthetic method of L-norvaline, take n-valeric acid as main starting material, make through following steps successively:
[0015] (1) bromo, the preparation of α-bromo n-valeric acid:
[0016] In the flask equipped with stirring, constant pressure dropping funnel, reflux condenser, calcium chloride drying tube, gas absorption device and thermometer, add n-valeric acid (20.4g, 0.2mol), and red phosphorus 0.62 g (0.02mol) (dried at 105°C for 2h), then dropwise added liquid bromine (33.6, 0.21mol) dried with an equal volume of concentrated sulfuric acid to the flask, after adding 1mL, stirred at room temperature until the color of bromine faded, and the liquid Pale yellow, HBr gas is absorbed with lye. Thereafter, continue stirring and adding bromine dropwise at room temperature. The rate of addition should be controlled so that the color of bromine can fade quickly. After the dropwise addition, the temperature is raised to about 80°C, and the reaction is continu...
Embodiment 2
[0023] D, the synthetic method of L-norvaline, take n-valeric acid as main starting material, make through following steps successively:
[0024] (1) bromo, the preparation of α-bromo n-valeric acid:
[0025] Add n-valeric acid (40.8g, 0.4mol) and red phosphorus 1.24 g (0.04mol) (dry at 105°C for 2h), then add dropwise liquid bromine (95.6g, 0.6mol) dried with an equal volume of concentrated sulfuric acid into the flask, add 1mL, stir at room temperature until the color of bromine fades, The liquid is pale yellow, and hydrogen bromide gas is absorbed with lye. Thereafter, continue to stir and add bromine dropwise at room temperature. The rate of addition should be controlled so that the color of bromine can fade quickly. Stop heating and stirring, let it stand overnight, distill off excess bromine, collect 132°C-136°C / 36kPa (25mmHg) fractions, and obtain 70.1g of light yellow transparent liquid, which is α-bromo-n-valeric acid, with a yield of 96.8 %.
[0026] (2) Ammonoly...
Embodiment 3
[0032] D, the synthetic method of L-norvaline, take n-valeric acid as main starting material, make through following steps successively:
[0033] (1) bromo, the preparation of α-bromo n-valeric acid:
[0034] In the flask equipped with stirring, constant pressure dropping funnel, reflux condenser, calcium chloride drying tube, gas absorption device and thermometer, add n-valeric acid (40.8g, 0.4mol) and 1mL of phosphorus tribromide, and heat up After reaching 80°C, liquid bromine (76.7 g, 0.48 mol) dried with an equal volume of concentrated sulfuric acid was added dropwise to the flask, and the dropping time was controlled within 3 h, and the stirring reaction was continued for 3 h. Stop heating and stirring, let it stand still, distill off excess bromine, collect 132°C-136°C / 36kPa (25mmHg) fractions, and obtain 68.0g of light yellow transparent liquid, which is α-bromo-n-valeric acid, with a yield of 94.0% .
[0035] (2) Ammonolysis, D, the preparation of L-norvaline:
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com