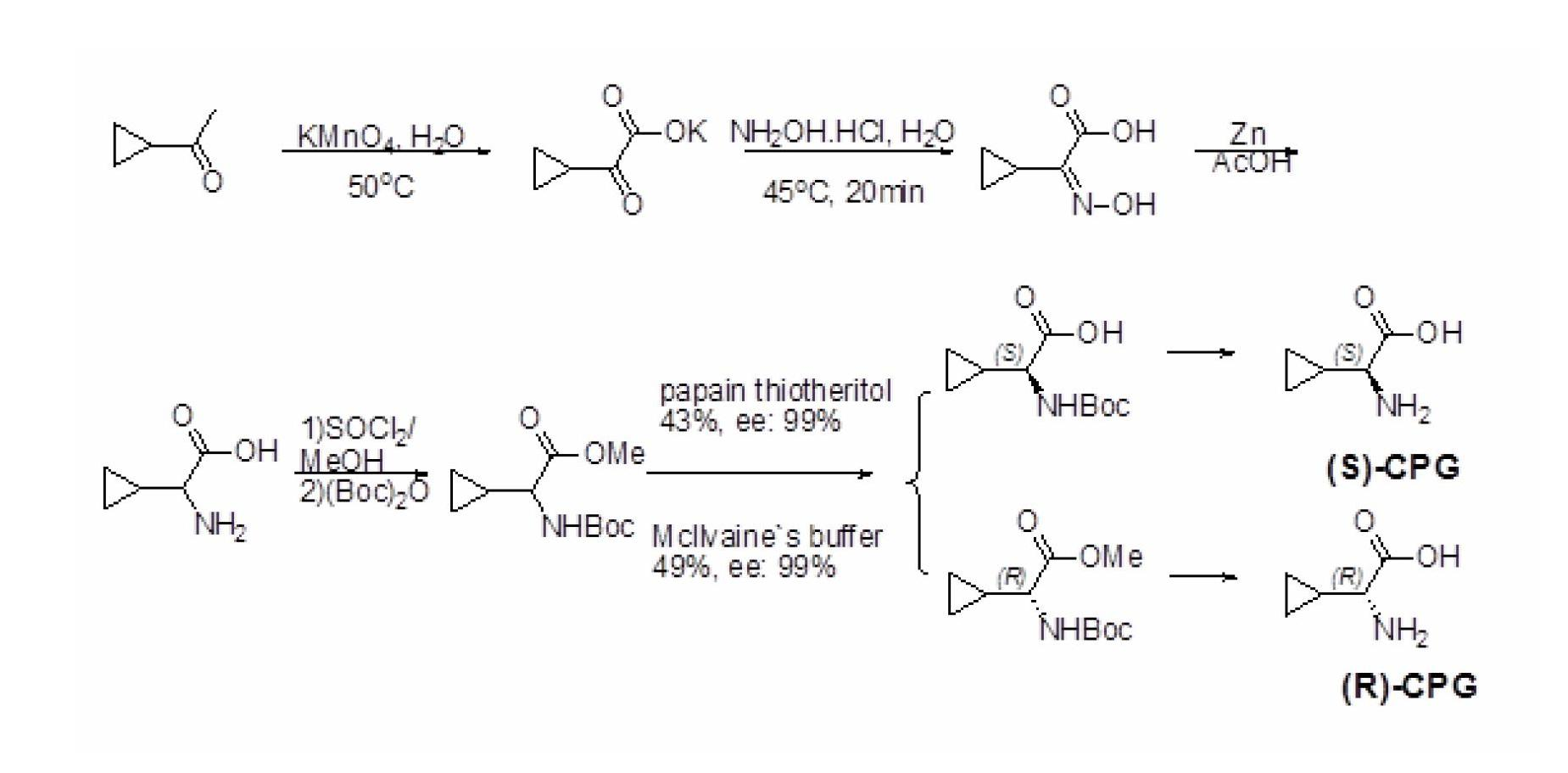
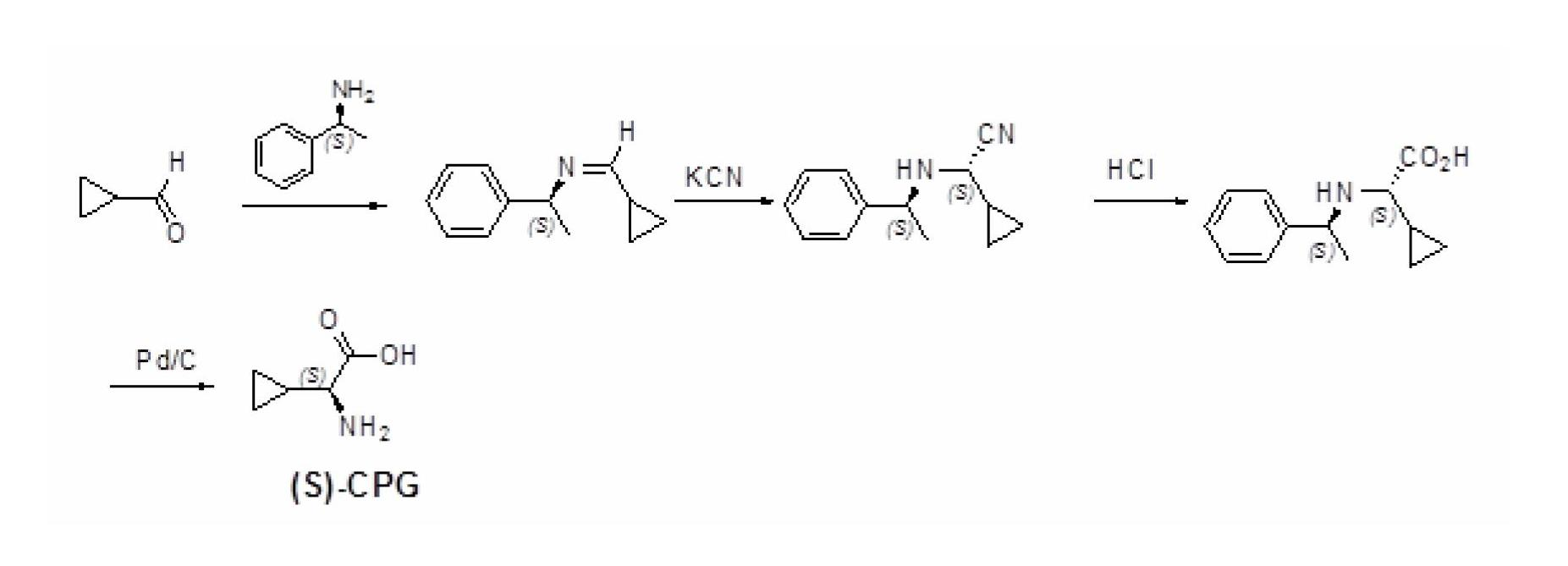
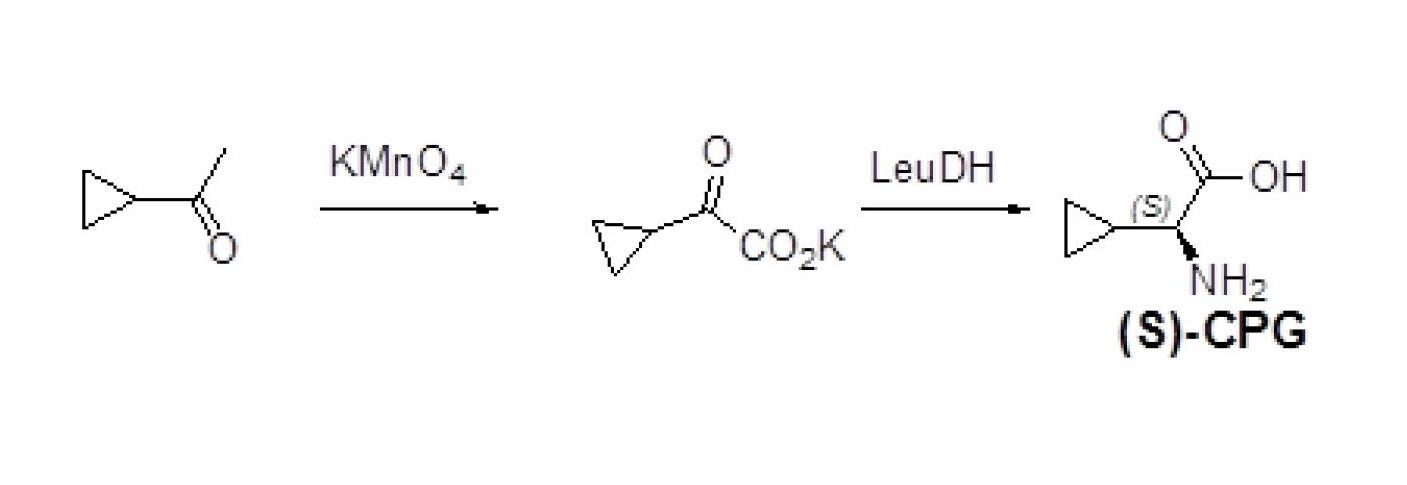
Method for preparing (S)-2-aminocyclopropylacetic acid
A technology of glycolic acid and amino ring, which is applied in the field of preparation of 2-aminocyclopropylacetic acid, can solve the problems of difficult industrial production, expensive raw materials, long routes, etc., and achieve the effect of simple and optimized synthetic process route and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Synthetic route see image 3 .
[0029] step 1
[0030] Add 60mL of water and sodium carbonate solid particles (138mg, 0.012eq.) into a 1L three-necked flask, stir to dissolve it, and then slowly add the substrate cyclopropyl methyl ketone (9.1g, 10.72mL, 108.2mmol), to The mixture was heated until the internal temperature reached 50°C. At this time, the aqueous solution of potassium permanganate (34.2g, 216.4mmol, 1500mL), which had been heated to 80°C and dissolved, was slowly added dropwise with a constant pressure dropping funnel until the addition was completed. Finally, the balloon was covered with the mouth of the bottle, and the reaction was carried out overnight at 50°C. After cooling the reaction solution to room temperature, add methanol (60mL) and stir for a few minutes, then filter with celite, wash with hot water once, adjust the pH value of the filtrate to 5-6, extract with DCM (6X), and use anhydrous sulfuric acid for the organic layer After the magne...
Embodiment 2
[0035] Synthetic route see image 3 .
[0036] step 1
[0037]Add 60mL of water and sodium hydroxide solid particles (48mg, 0.012eq.) into a 1L three-necked flask, stir to dissolve, and then slowly add the substrate cyclopropyl methyl ketone (9.1g, 10.72mL, 108.2mmol), Heat the mixture until the internal temperature reaches 60°C, then slowly add a solution of potassium permanganate (51.3g, 324.6mmol, 1500mL) that has been heated to 50°C dropwise with a constant pressure dropping funnel until the addition is complete Finally, the balloon was covered with the mouth of the bottle, and the reaction was carried out overnight at 50°C. After cooling the reaction solution to room temperature, add methanol (60mL) and stir for a few minutes, then filter with celite, wash with hot water once, adjust the pH value of the filtrate to 5-6, extract with DCM (6X), and use anhydrous sulfuric acid for the organic layer After the magnesium was dried, it was concentrated under reduced pressure,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com