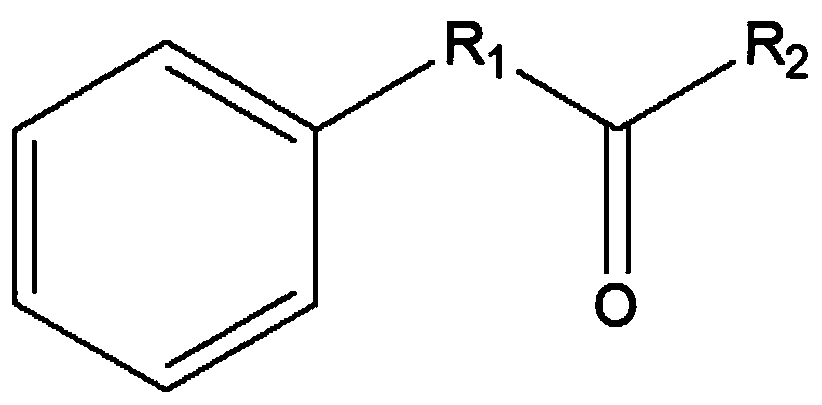
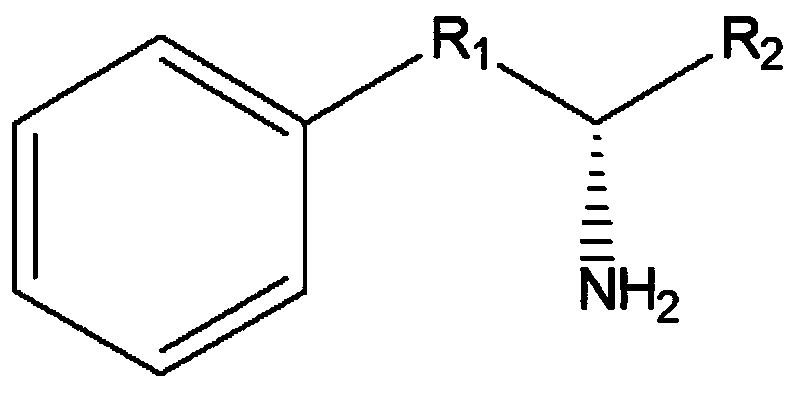
Leucine dehydrogenase mutant and application thereof to synthesis of aromatic chiral amine
A technology of leucine dehydrogenase and mutants, applied in applications, enzymes, oxidoreductases, etc., can solve problems such as low catalytic activity and affect the conversion rate of 4-phenyl-2-butylamine, and achieve catalytic activity high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Example 1: Preparation and expression of different leucine dehydrogenase mutants
[0085] Specific steps are as follows:
[0086] Chemically synthesize the gene of leucine dehydrogenase encoding amino acid sequence as shown in SEQ ID NO.1 (the nucleotide sequence of the gene is as shown in SEQ ID NO.2); With reference to the literature "Chen F F, Liu Y Y, Zheng G W , etal.Asymmetric Amination of Secondary Alcohols by using a Redox-Neutral Two-Enzyme Cascade[J].ChemCatChem,2015,7(23):3838-3841."The 70th lysine of leucine dehydrogenase Mutation to serine, asparagine at position 263 to leucine, so that leucine dehydrogenase obtains the activity of aliphatic ketones (the 70th position here corresponds to the 77th position in the literature, and the 263rd position here is the Corresponding to the 270th position in the literature), obtain the leucine dehydrogenase mutant (amine dehydrogenase) whose amino acid sequence is shown in SEQ ID NO.3 and the leucine whose encoding am...
Embodiment 2
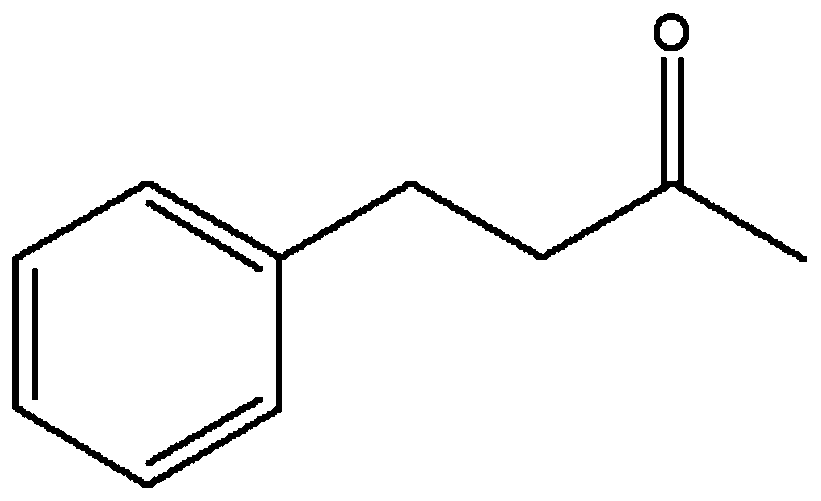
[0112] Example 2: Catalytic activity of different leucine dehydrogenase mutants to 4-phenyl-2-butanone
[0113] Specific steps are as follows:
[0114] in NH 4 Cl-NH 4 Add 4-phenyl-2-butanone (5mmol / L) and NADH (0.2mmol / L) to OH buffer (1mol / L, pH 9.0) to obtain a reaction system; keep the reaction system at 30°C for 2min Add 20ul of wild type, mutant A115G, T136A, A115G / T136A, A115G / T136A / L42A, A115G / T136A / T45A, A115G / T136A / T114A, A115G / T136A / E116A and A115G / T136A / L42G obtained in Example 1 Concentrate pure enzyme to start the reaction, the control group does not contain concentrated enzyme solution, and other components are the same; the reaction is carried out at 30°C for 5min, and the absorbance change at 340nm is recorded every 10s to obtain wild type, mutant A115G, T136A, A115G / Catalytic activity of T136A, A115G / T136A / L42A, A115G / T136A / T45A, A115G / T136A / T114A, A115G / T136A / E116A and A115G / T136A / L42G on 4-phenyl-2-butanone; catalytic activity (U / g) = Ew × V × 1000 / 62...
Embodiment 3
[0116] Embodiment 3: the catalytic activity of different leucine dehydrogenase mutants to phenylacetone
[0117] Specific steps are as follows:
[0118] Add NH in Tris-HCL buffer (100mmol / L, pH 9.0) 4Cl (1mol / L), phenylacetone (5mmol / L) and NADH (0.2mmol / L) to obtain a reaction system; after incubating the reaction system at 30°C for 2min, add 20ul of the wild type and mutant obtained in Example 1 The concentrated pure enzymes of A115G / T136A, A115G / T136A / L42A, and A115G / T136A / L42G start the reaction. The control group does not contain concentrated enzyme solution, and the other components are the same; the reaction is carried out at 30°C for 5 minutes, and the temperature at 340nm is recorded every 10s. The absorbance change of wild type, mutant A115G / T136A, A115G / T136A / L42A, A115G / T136A / L42G is obtained the catalytic activity of phenylacetone; Catalytic activity (U / g)=Ew×V×1000 / 6220 / L ×The protein concentration of the concentrated enzyme solution; where, Ew is the absorbanc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com