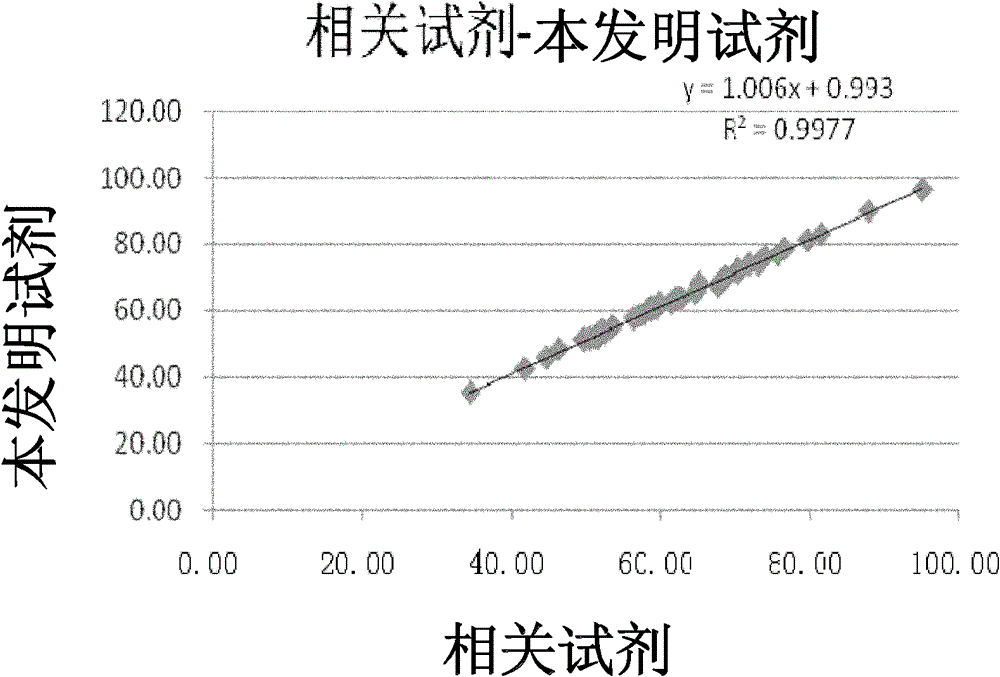
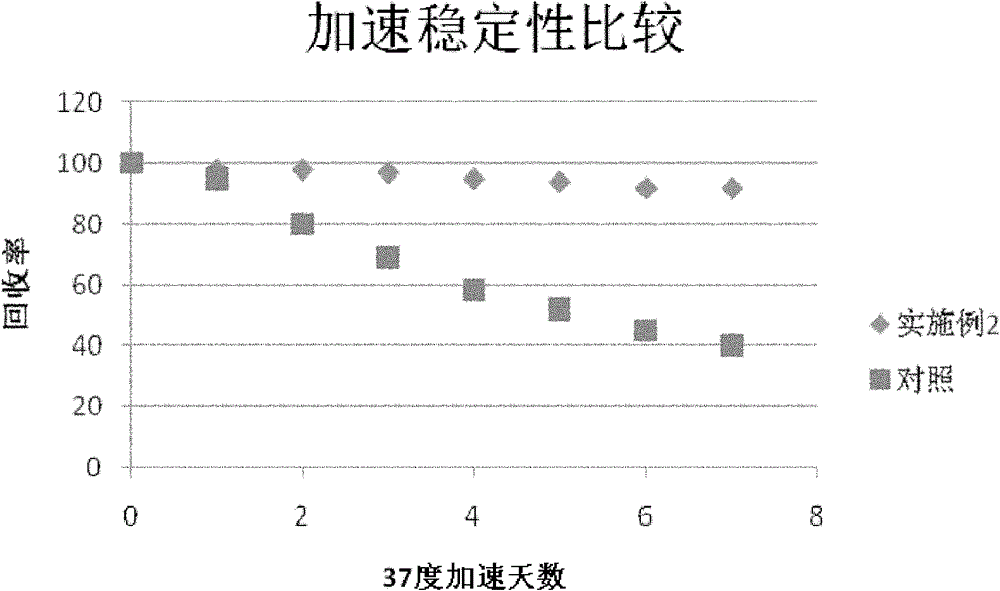
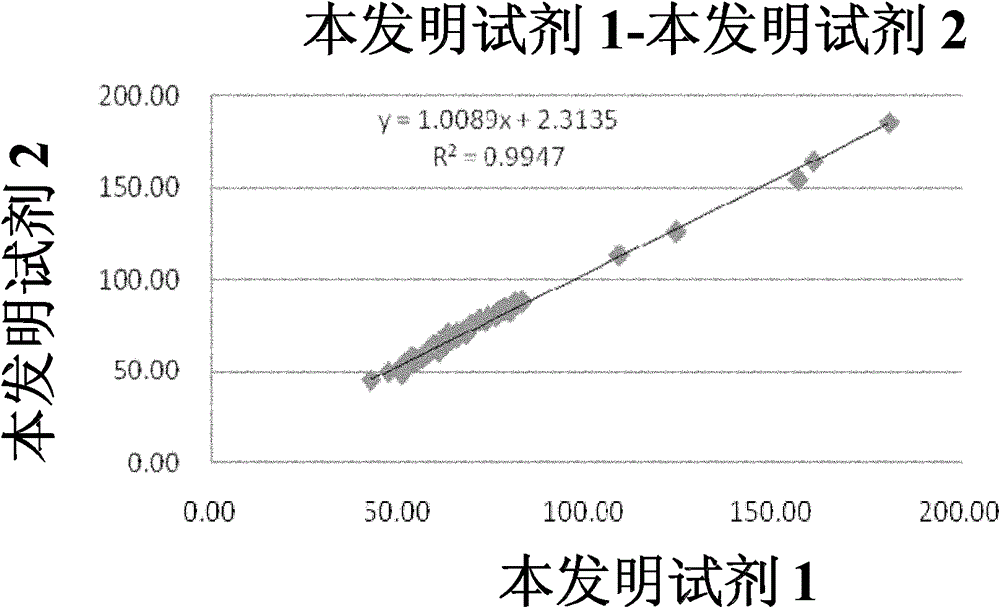
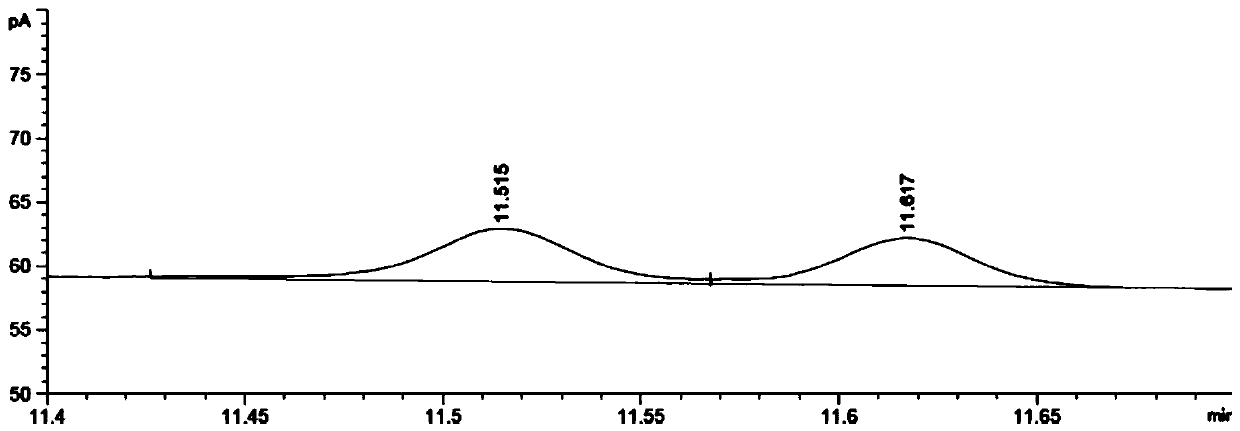
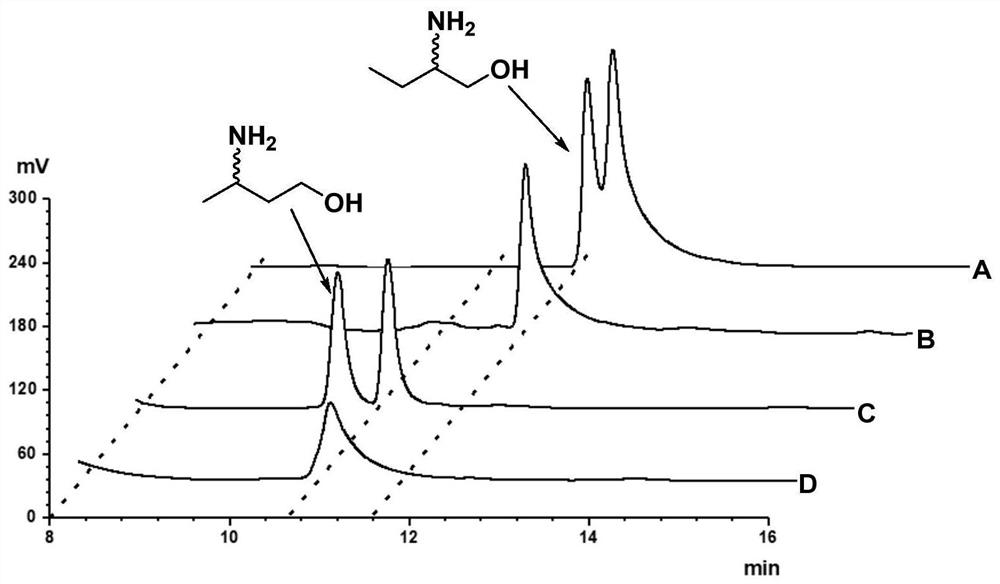
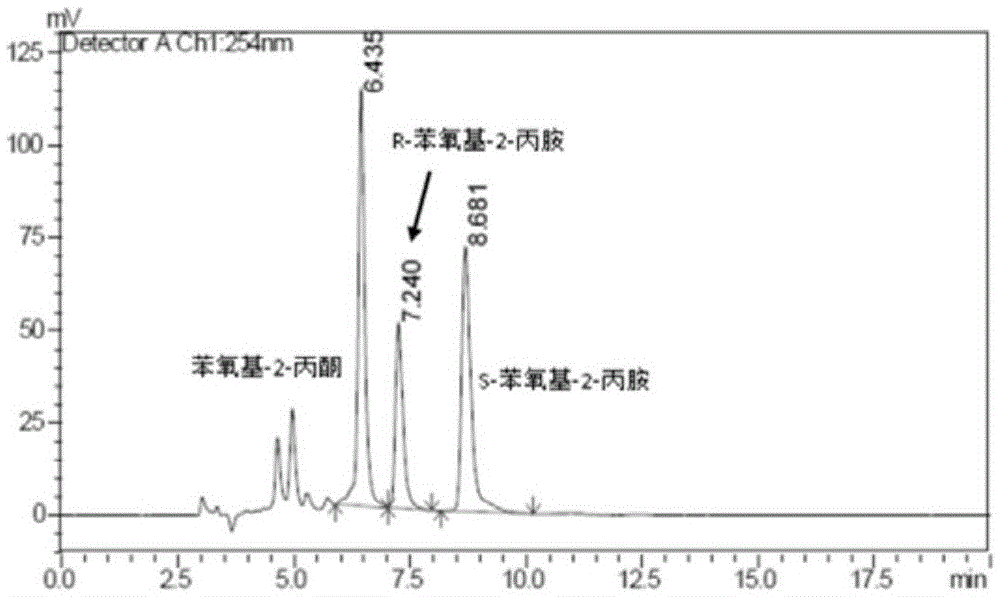
Patents
Literature
33 results about "Amine dehydrogenase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
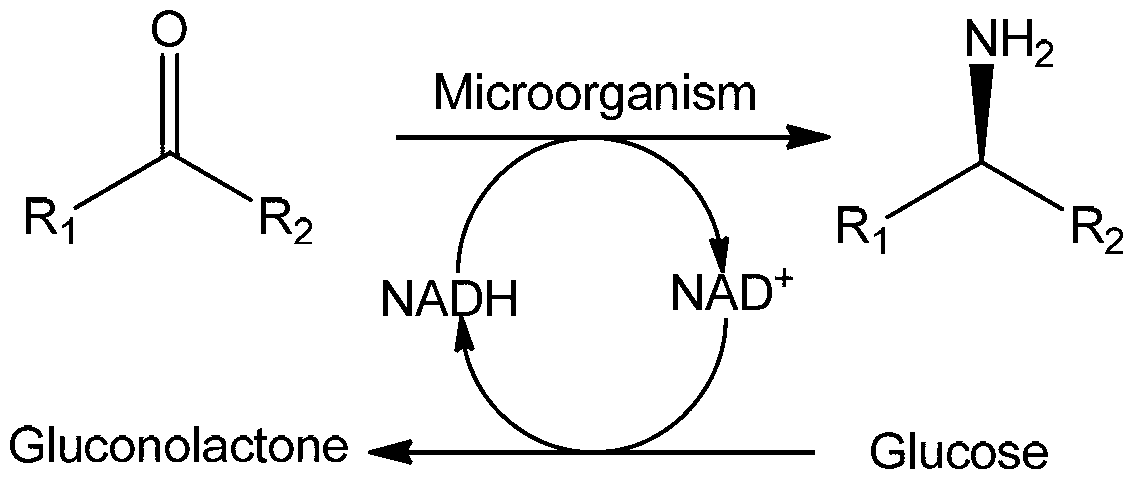
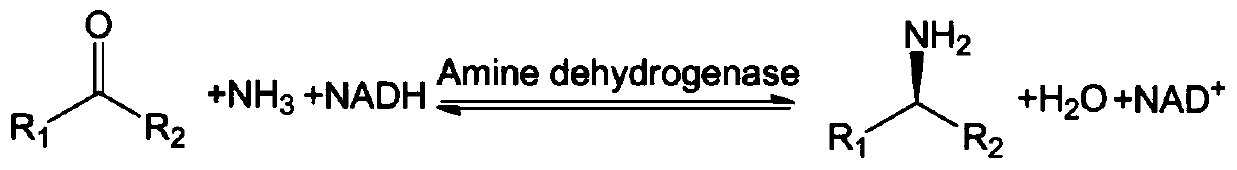
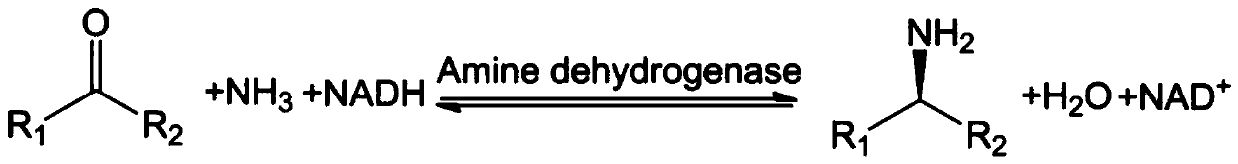
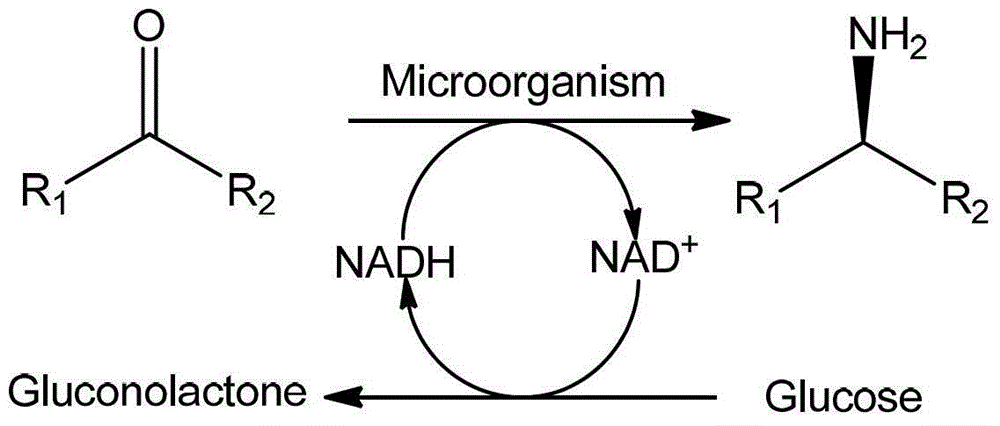
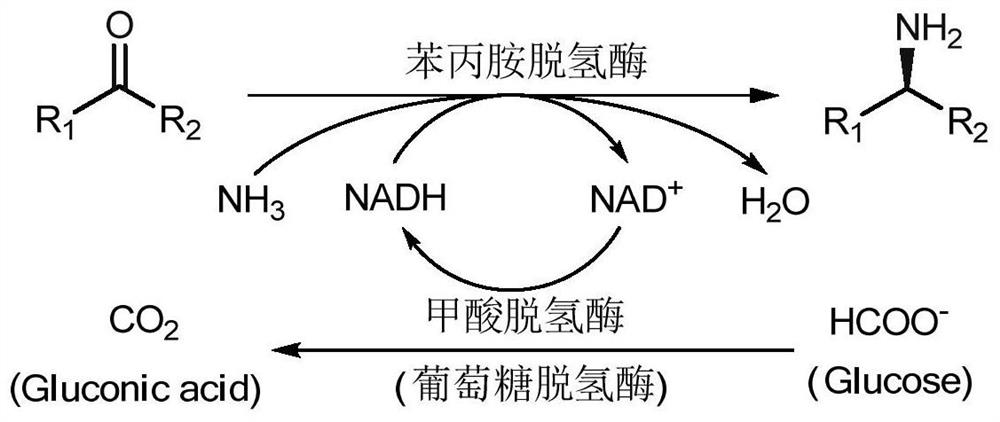
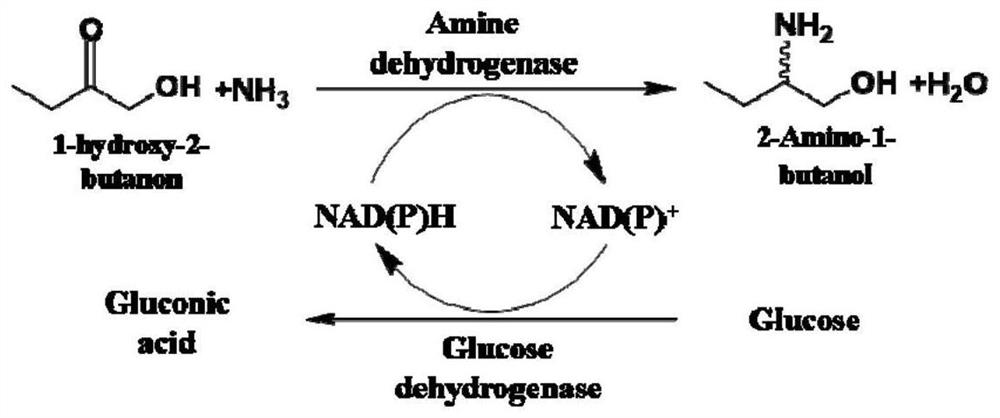
Amine Dehydrogenase (EC 1.4.99.3), also known as methylamine dehydrogenase (MADH), is a tryptophan tryptophylquinone-dependent (TTQ-dependent) enzyme that catalyzes the oxidative deamination of a primary amine to an aldehyde and ammonia.
Engineered Amine Dehydrogenases and Methods of Use Thereof
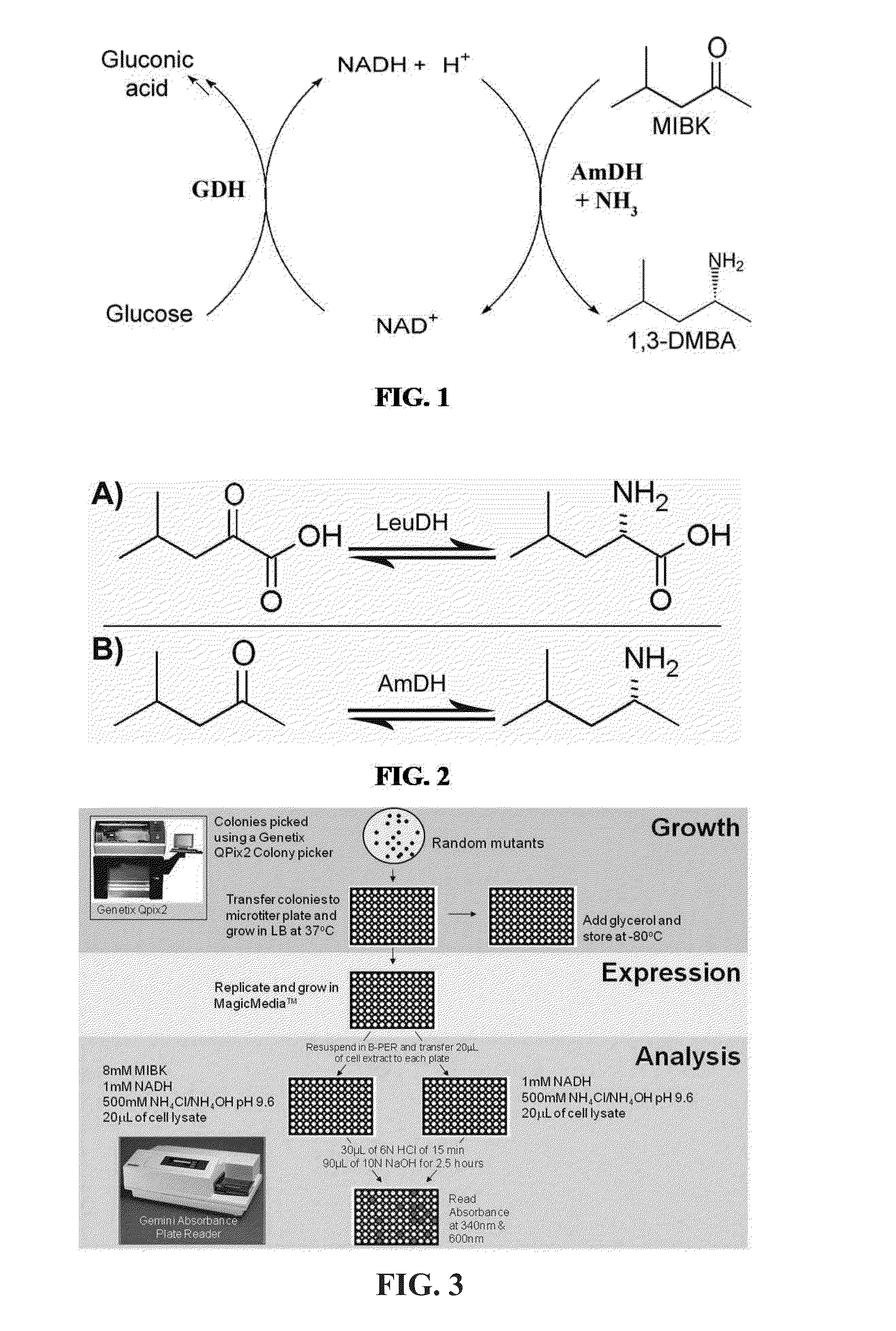
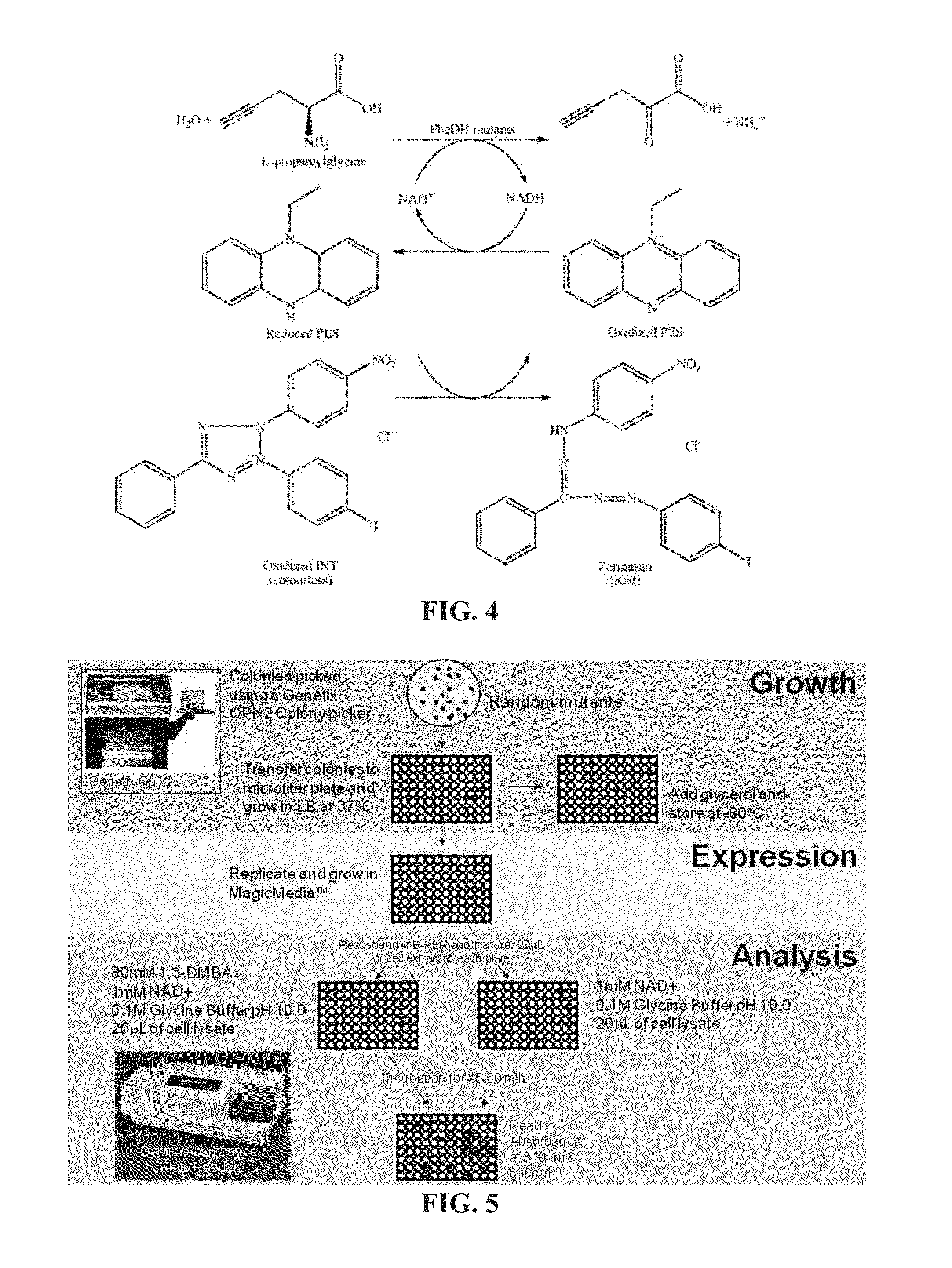
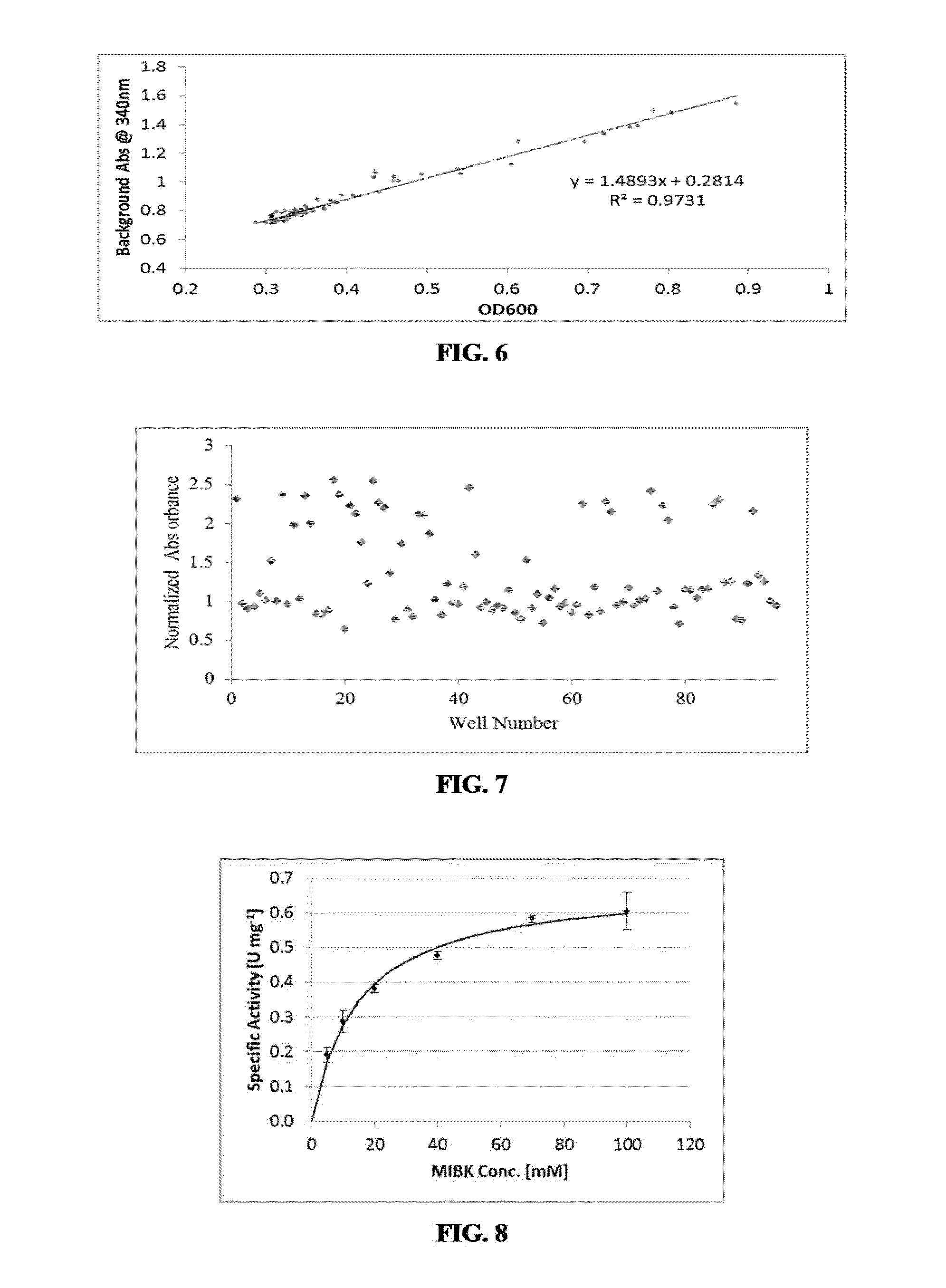
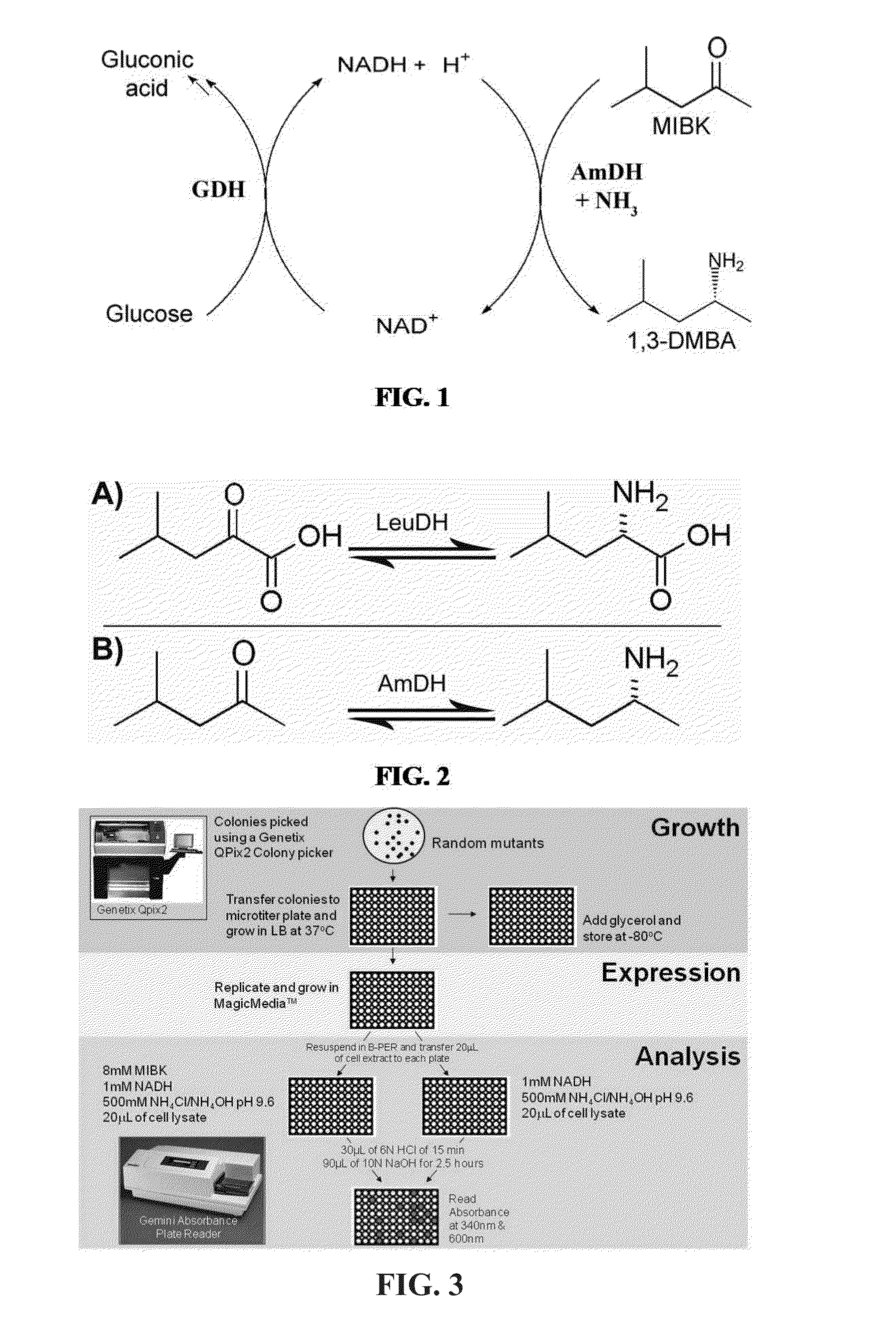
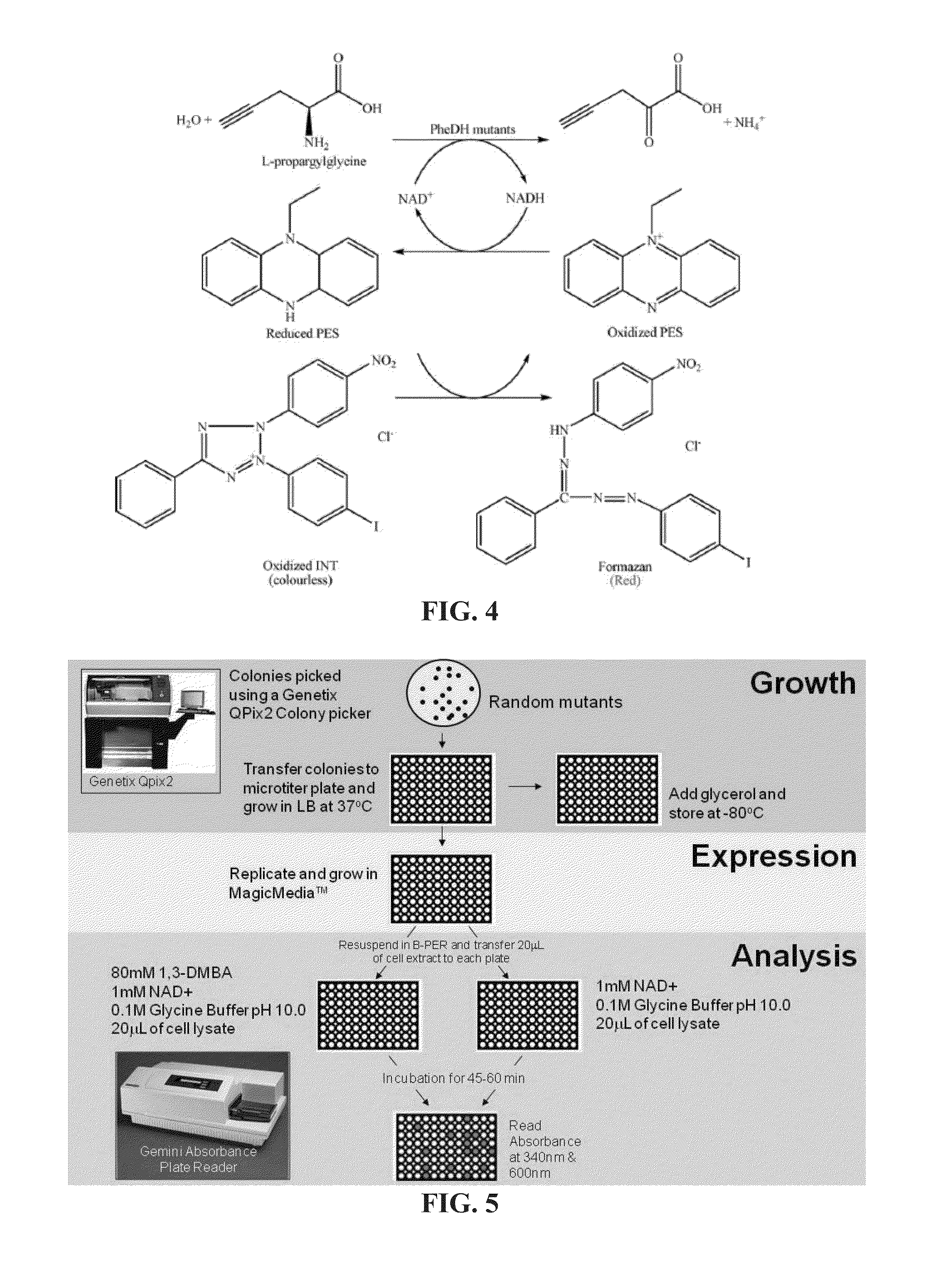
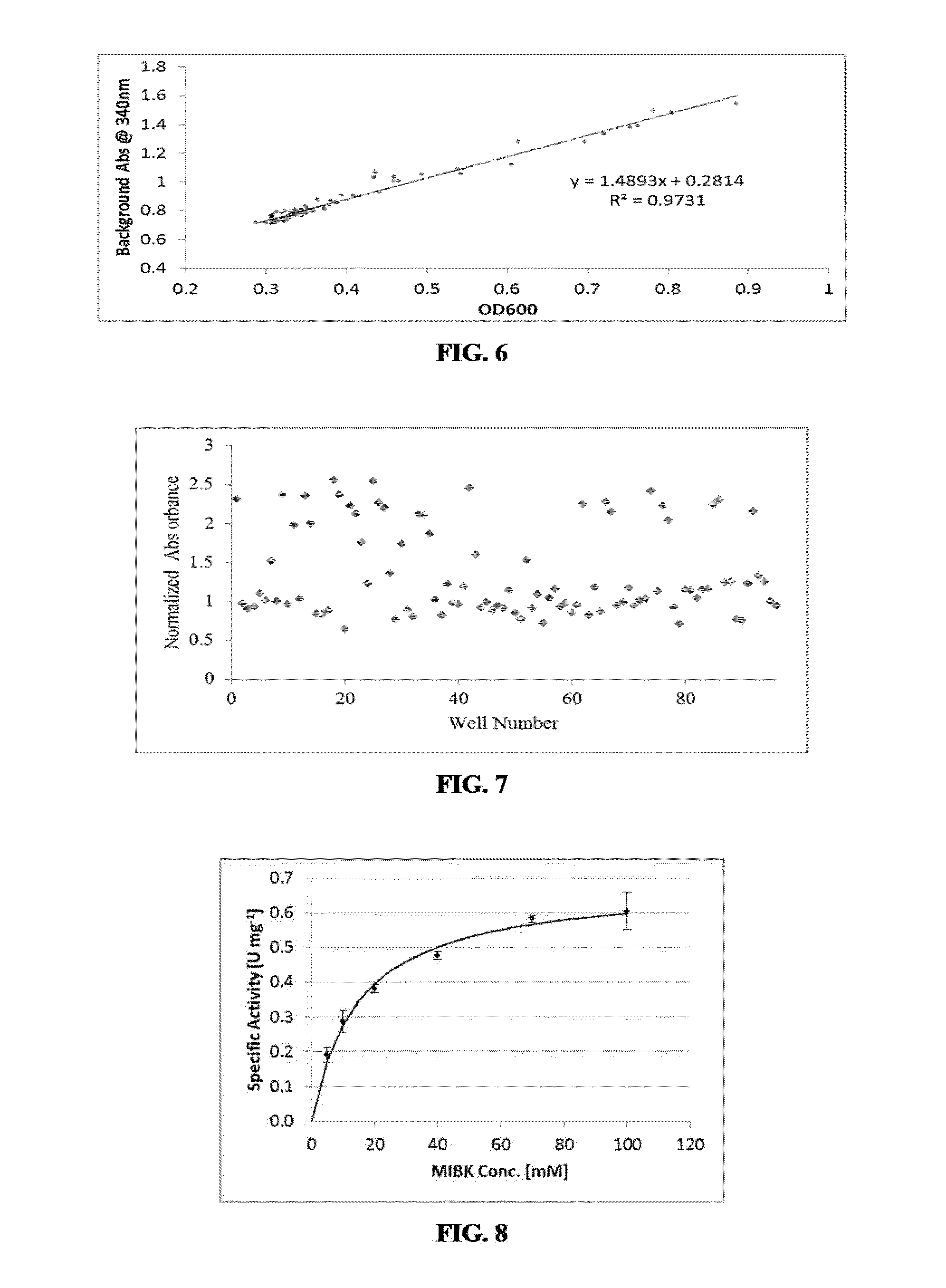
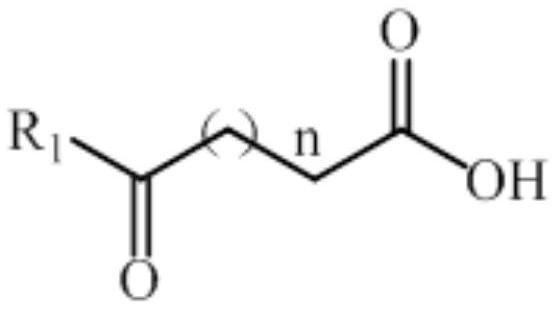
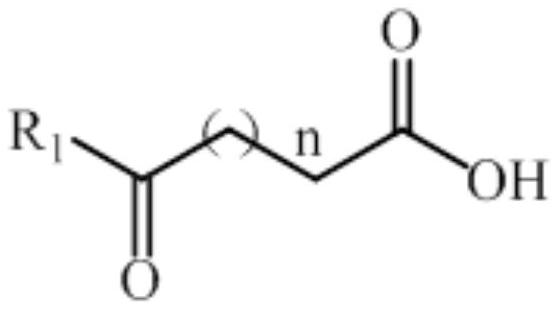
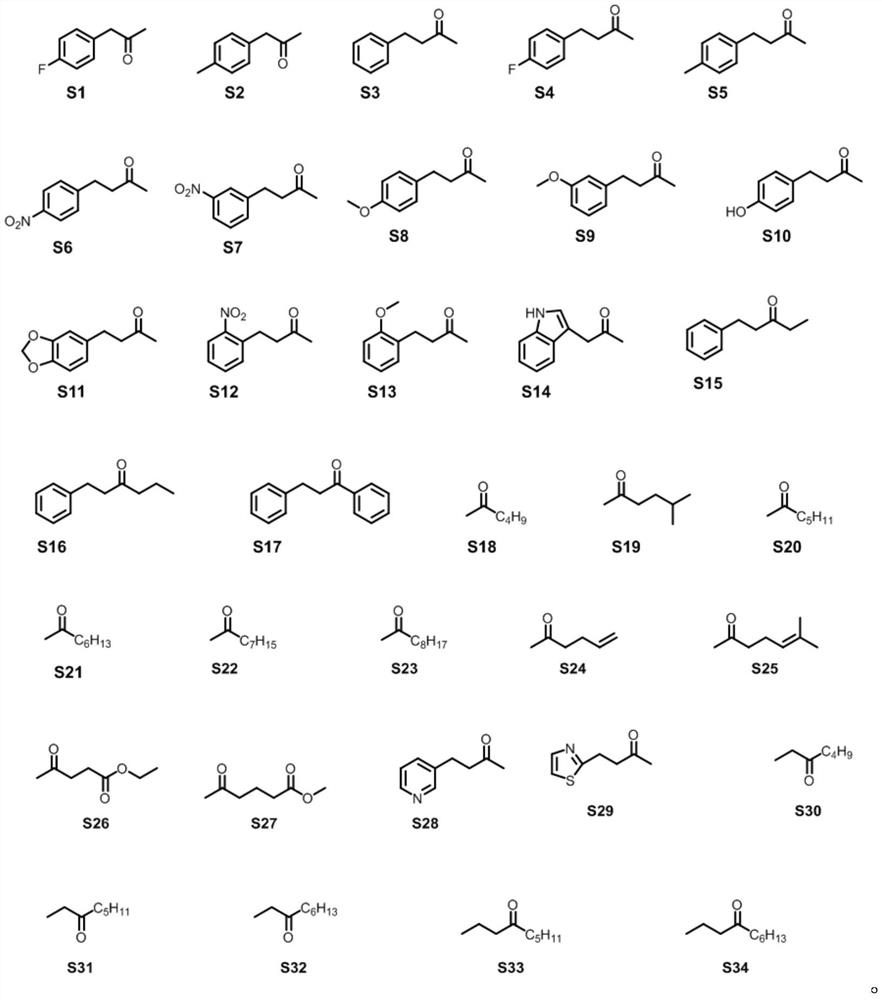
Non-naturally occurring amine dehydrogenases (AmDH) and methods of use thereof the produce chiral amines are disclosed. The AmDH are variants of amino acid dehydrogenases. AmDH based on phenylalanine, leucine, and valine scaffolds are provided. The AmDH typically have one, two, three, four, or more amino acid alterations relative to the scaffold. The alterations to the scaffold result in an enzyme that accepts the analogous ketone, such as methyl isobutyl ketone (MIBK), instead of the wild-type α-keto acid. Chimeric AmDH are also disclosed. The chimeras are fusion proteins that include a substrate binding domain from a first AmDH and a cofactor binding domain from a second AmDH. In a preferred embodiment, one of the domains is from a PheDH-based AmDH and one of the domains is from a LeuDH-based AmDH.
Owner:GEORGIA TECH RES CORP
Method for preparing chiral amine through asymmetric reduction under catalysis of marine strain
InactiveCN103224963AImprove responseOptimizationMicroorganism based processesFermentationMicroorganismKetone
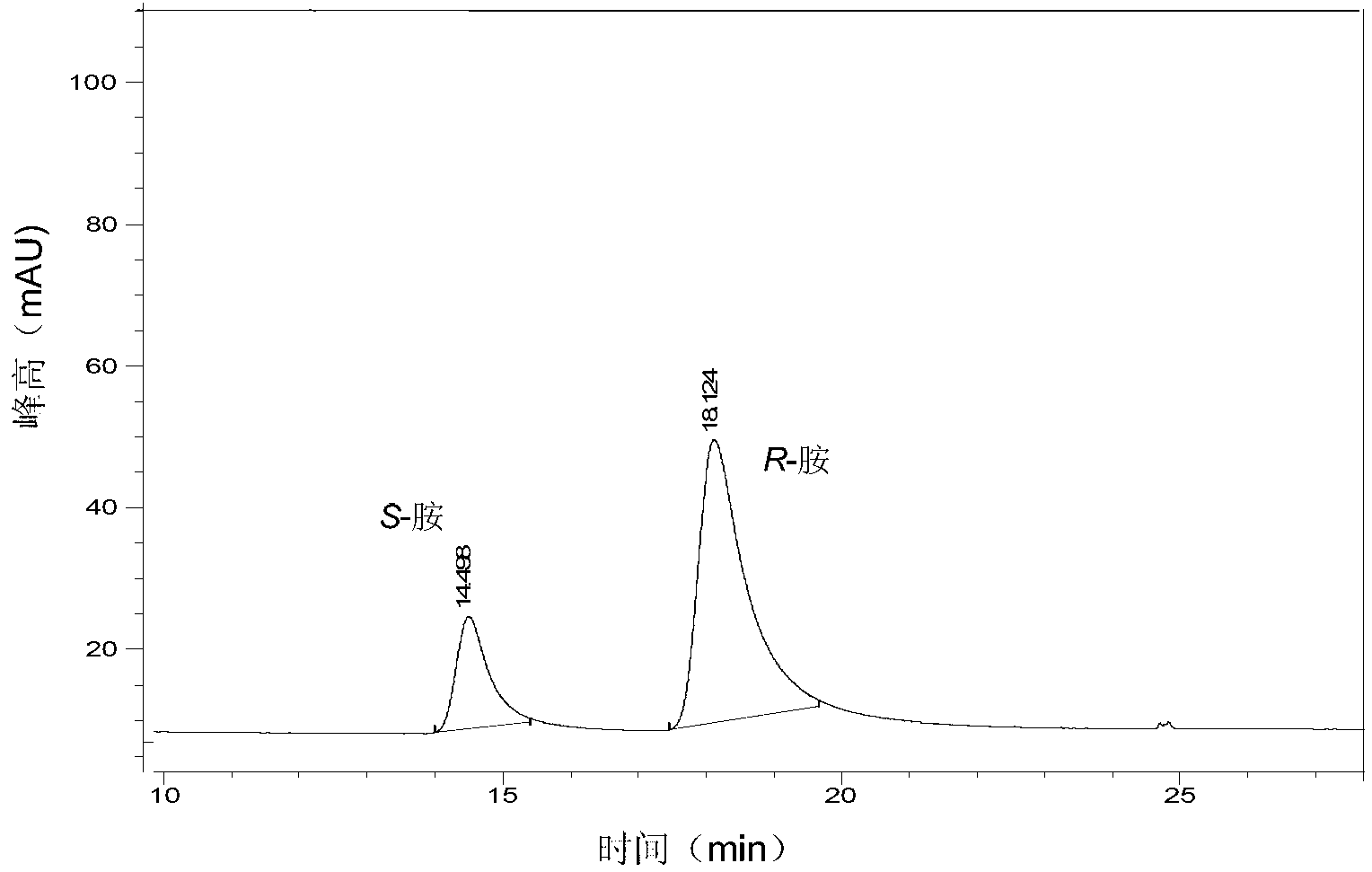
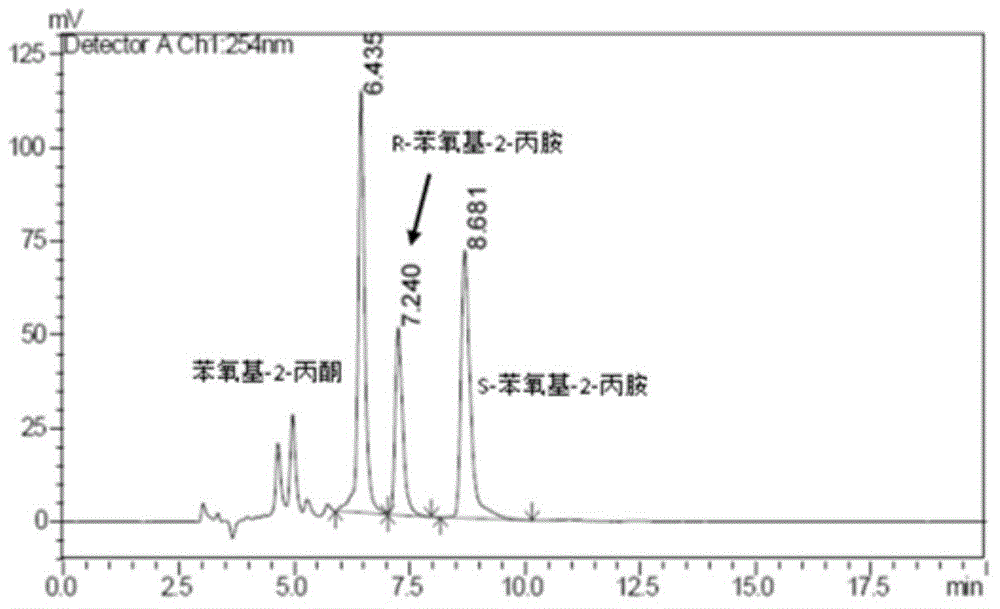
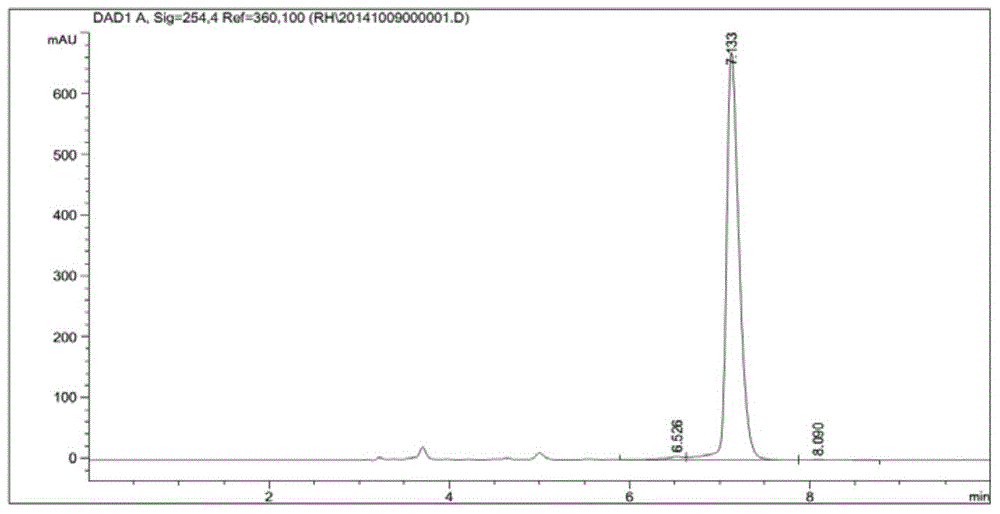
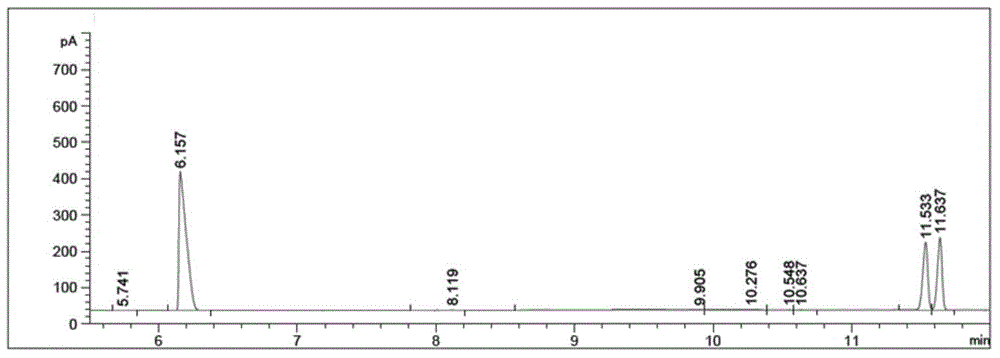
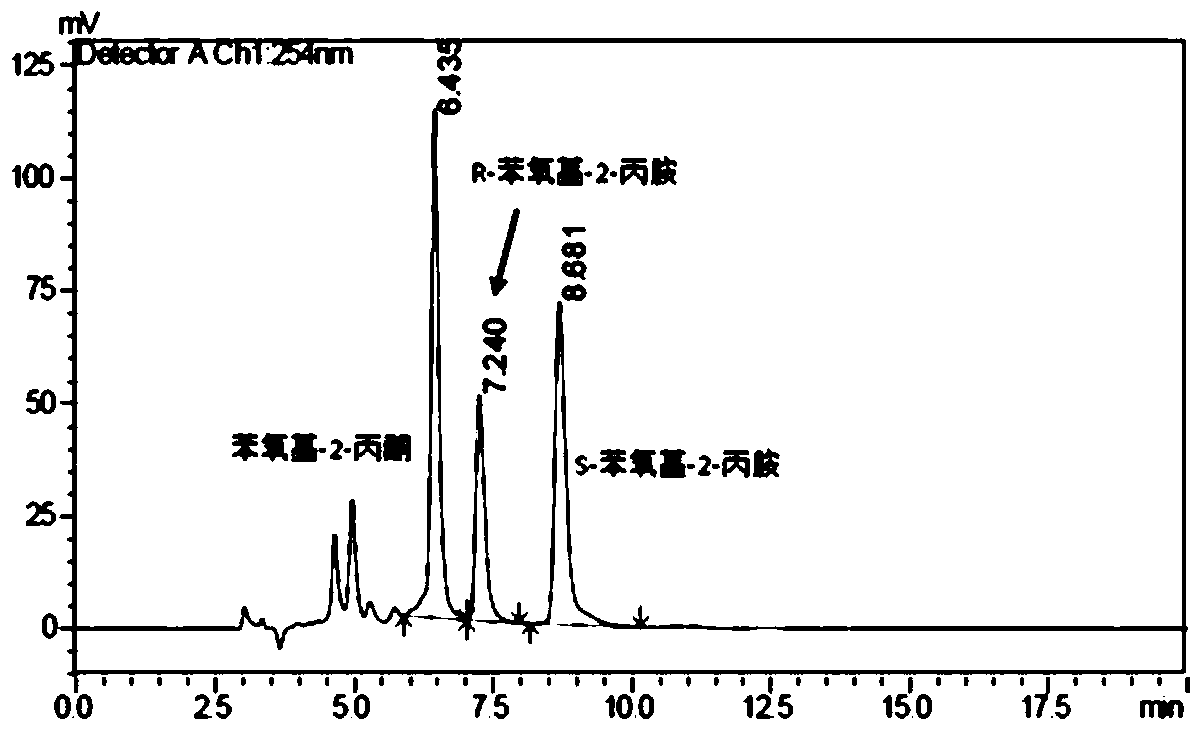
The invention provides a method for preparing chiral amine through asymmetric reduction under the catalysis of a marine strain, and relates to chiral amine. The method comprises the following steps: inoculating a strain into a 216L culture medium, culturing, then centrifuging the obtained fermentation liquid to obtain cells, and redissolving and washing with a buffer solution, thus preparing cell sap; and adding ketone used as a substrate into the obtained cell sap, then adding an amino donor and a cosubstrate for cyclic regeneration of coenzyme, and performing asymmetric reduction and amination under the catalysis of the cells, thus obtaining the chiral amine product. The new strain which contains NADH (nicotinamide adenine dinucleotide)-dependent amine dehydrogenase and is different from strains reported before is obtained through the screening of marine microorganisms, and the reaction system and reaction conditions of preparing chiral amine through the asymmetric reduction and amination of ketone under the catalysis of full cells of the strain are optimized. The optical purity of the obtained chiral amine product is up to 90% or above, and the yield is up to 70% or above. The method provided by the invention is convenient to operate, has the advantages of high optical purity of the product, high yield and the like, is simple in equipment and has better industrial application prospects in the field of preparing chiral amine under biological catalysis.
Owner:XIAMEN UNIV
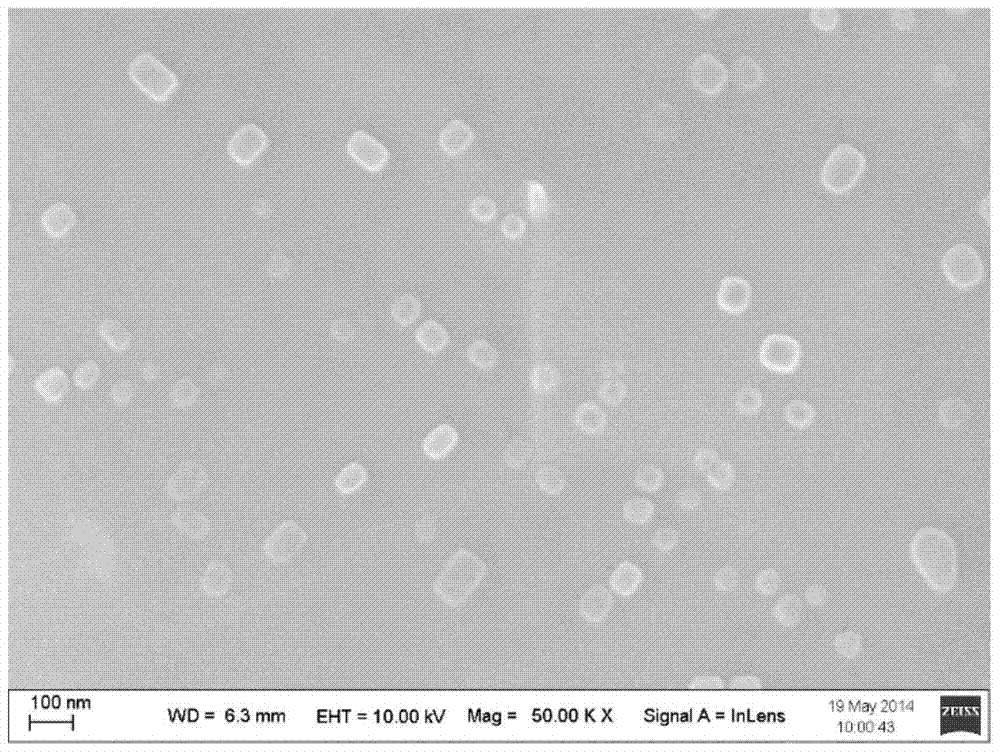
Polyethylene imine-titanium oxide embedded with amine dehydrogenase and preparation method of polyethylene imine-titanium oxide
ActiveCN104404026AReduce leak rateGood leak rateOn/in organic carrierOn/in inorganic carrierHydrogenTitanium
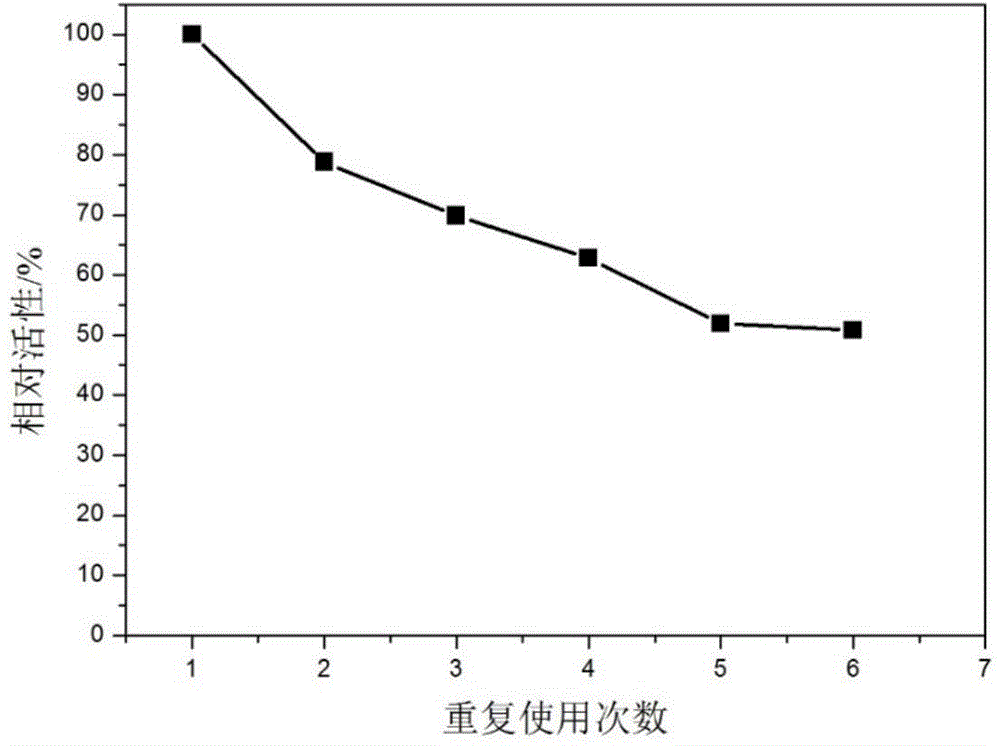
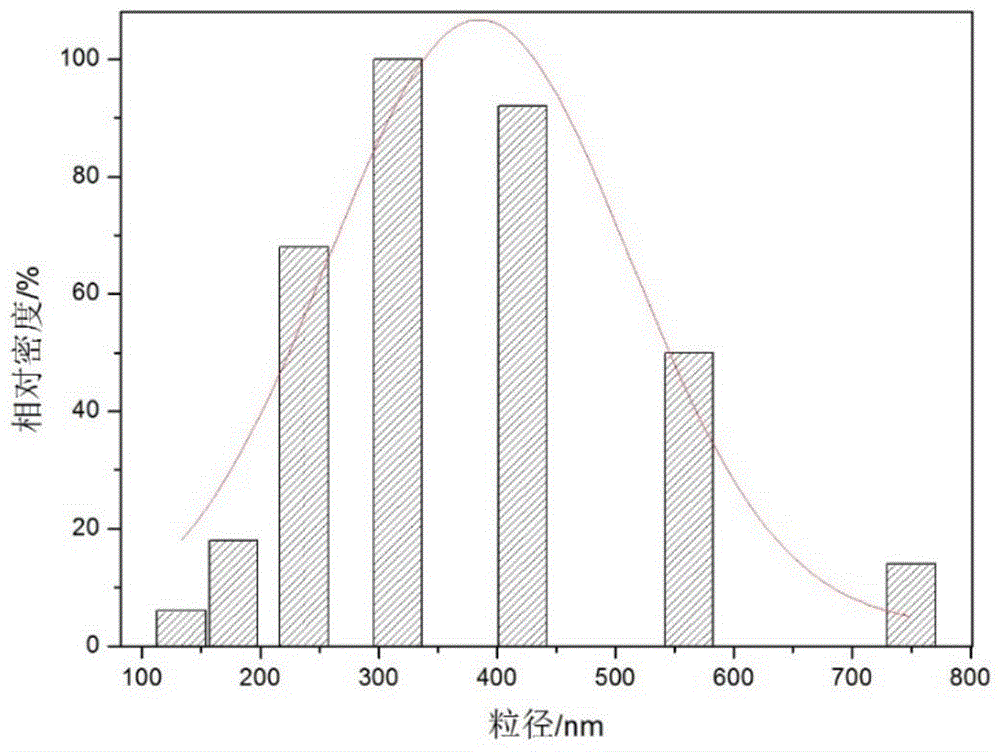
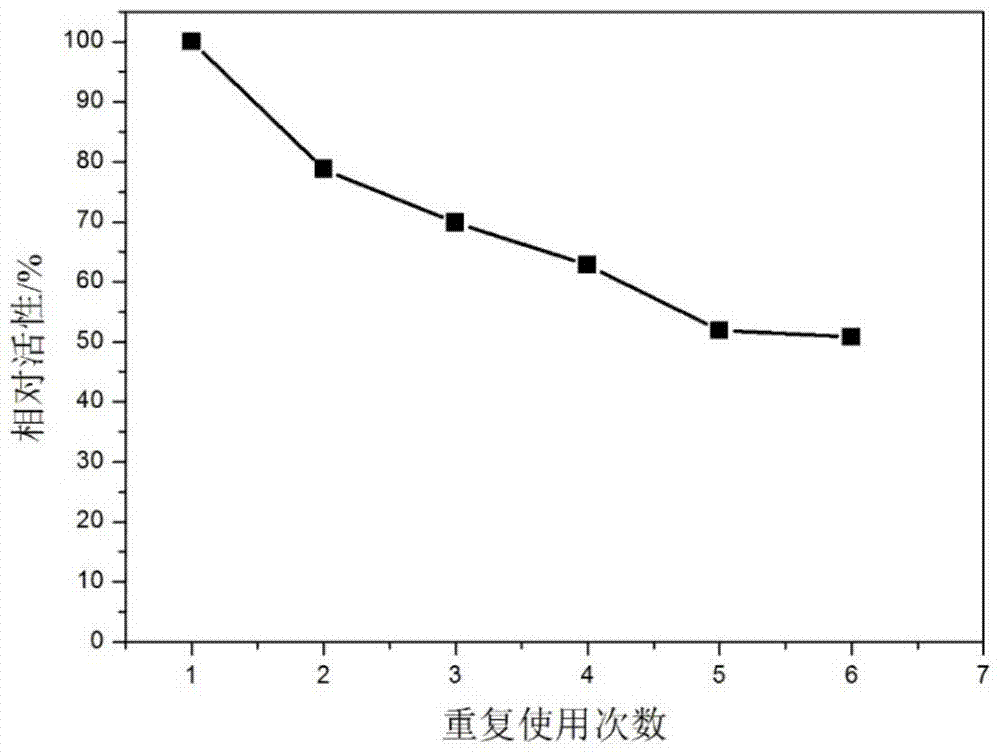
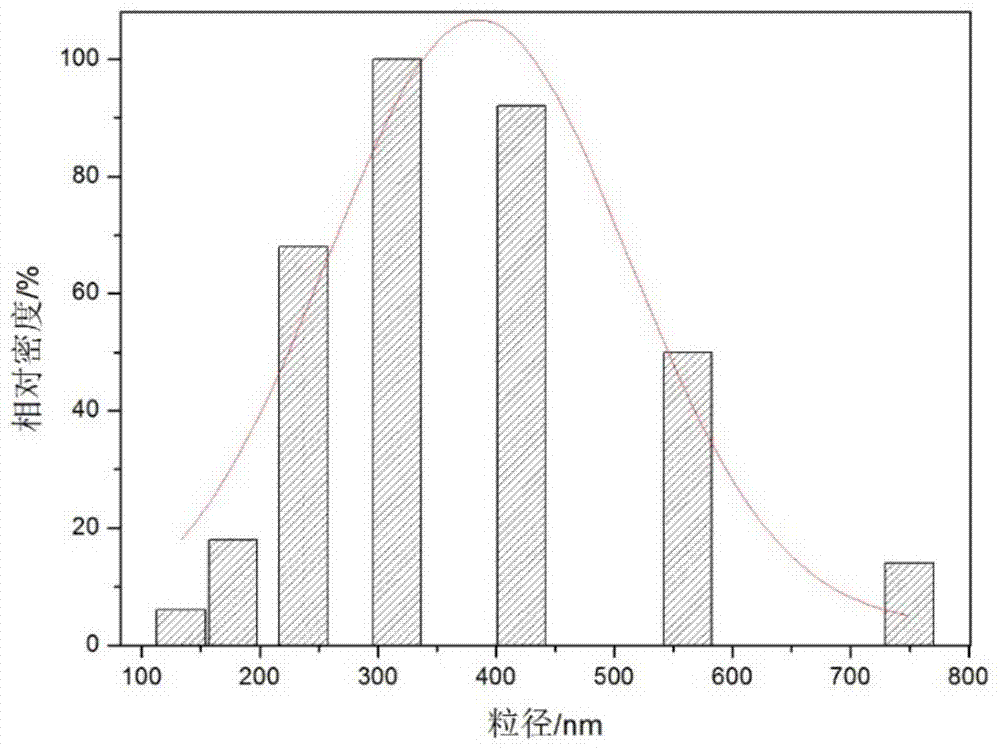
The invention relates to polyethylene imine-titanium oxide embedded with amine dehydrogenase and a preparation method of the polyethylene imine-titanium oxide, and belongs to the technical field of immobilized enzymes. A polyethylene imine (PEI)-titanium oxide immobilized particle embedded with the amine dehydrogenase has a grain size of 100-600 nm, wherein polyethylene imine has catalyzing and templating double effects in the immobilization process, namely the PEI promotes the formation of titanium oxide through an electrostatic and hydrogen bonding effect between an amino group in a molecule and a titanium precursor molecule; through a polyethylene imine structure, a template can also be induced, and the formation of a titanium oxide particle are regulated and controlled. The preparation method of the polyethylene imine-titanium oxide comprises the following steps: configuring the PEI (straight chain and branched chain) as well as mixed liquid containing the amine dehydrogenase; configuring a precursor II-dihydroxybis (2-hydroxypropanoato) titante diammonium (Ti-BALDH) solution of titanium; dropwise adding the precursor solution of the titanium into the PEI and the mixed liquid containing the amine dehydrogenase, stirring and generating the PEI-titanium oxide immobilized particle embedded with the amine dehydrogenase. The polyethylene imine-titanium oxide and the preparation method thereof have the advantages that the preparation conditions are mild, the process is simple and is easy to implement, the obtained immobilized particle embedded with the amine dehydrogenase has strong immobilization capacity and good reuse stability, and the temperature stability of the amine dehydrogenase can be obviously improved.
Owner:XIAMEN UNIV
Amine dehydrogenase electrode and preparation method and application thereof
ActiveCN109813781AGood biocompatibilityImprove electronic performanceMaterial nanotechnologyMicrobiological testing/measurementConductive polymerEnzyme electrode
The invention discloses an amine dehydrogenase electrode and a preparation method and application thereof, and relates to the technical field of biosensors, in particular to an enzyme electrode, an enzyme biosensor and a preparation method and application of the electrode. The electrode comprises a substrate electrode, an electron conducting layer and an amine dehydrogenase layer, the effective immobilization of enzyme molecules and the electron transfer between an enzyme and the electrode can be achieved, and the catalytic activity of the enzyme molecules and the sensitivity of the sensor areimproved. With conductive polymer polyethyleneimine-modified graphene and carbon nanotubes with good biocompatibility as an electrode material, the enzyme electrode has the advantages of simple manufacturing method, high detection sensitivity, quickness, accuracy and good stability and repeatability, and has good application prospects.
Owner:XIAMEN UNIV
Amine dehydrogenase mutant with improved thermal stability, construction method and application of genetic engineering bacteria
ActiveCN110846291AImprove thermal stabilityImprove stabilityOxidoreductasesFermentationRegioselectivityMutant
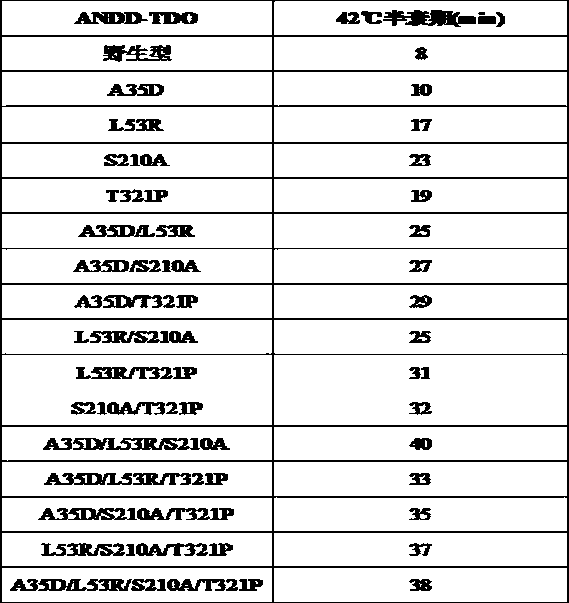
The invention belongs to the technical field of biology, in particular to an amine dehydrogenase mutant with improved thermal stability, a construction method and an application of genetic engineeringbacteria. The amine dehydrogenase mutant comprises 4 single-site mutants and 11 combined mutants, and compared with wild-type amine dehydrogenase, the single-site mutants and the combined mutants have longer half-life period at 42 DEG C; in particular, the combined mutants show a superposition effect of thermal stability of the single-site mutants, and the half-life period is about 5 times that of the wild-type amine dehydrogenase. The amine dehydrogenase mutant obtained by the construction method has better thermal stability, shows excellent stereoselectivity, regioselectivity and catalyticactivity when being catalyzed for synthesis of chiral amine at higher temperature, and has a better application prospect.
Owner:SUZHOU INST OF BIOMEDICAL ENG & TECH CHINESE ACADEMY OF SCI
Engineered amine dehydrogenases and methods of use thereof
Non-naturally occurring amine dehydrogenases (AmDH) and methods of use thereof the produce chiral amines are disclosed. The AmDH are variants of amino acid dehydrogenases. AmDH based on phenylalanine, leucine, and valine scaffolds are provided. The AmDH typically have one, two, three, four, or more amino acid alterations relative to the scaffold. The alterations to the scaffold result in an enzyme that accepts the analogous ketone, such as methyl isobutyl ketone (MIBK), instead of the wild-type α-keto acid. Chimeric AmDH are also disclosed. The chimeras are fusion proteins that include a substrate binding domain from a first AmDH and a cofactor binding domain from a second AmDH. In a preferred embodiment, one of the domains is from a PheDH-based AmDH and one of the domains is from a LeuDH-based AmDH.
Owner:GEORGIA TECH RES CORP
Amine dehydrogenase mutant, enzyme preparation, recombinant vector, recombinant cell, preparation methods therefor and application of amine dehydrogenase mutant
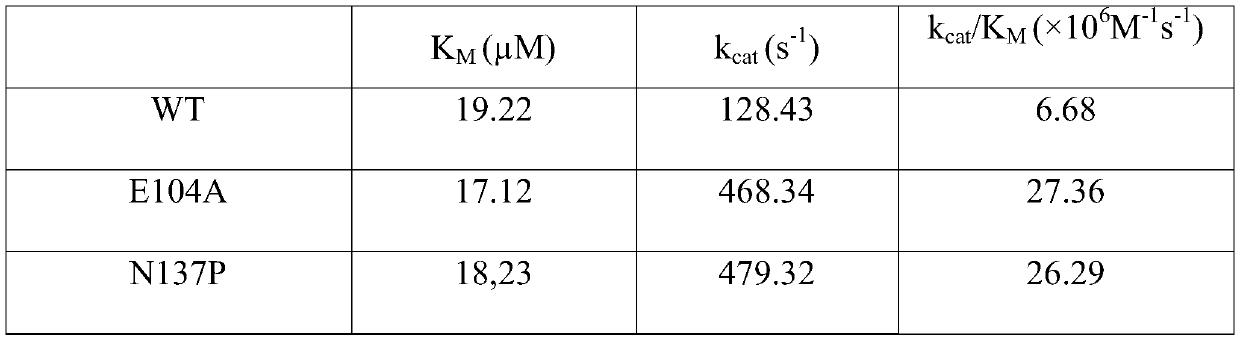
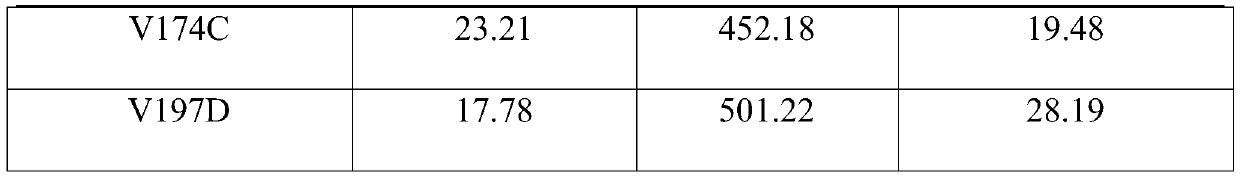
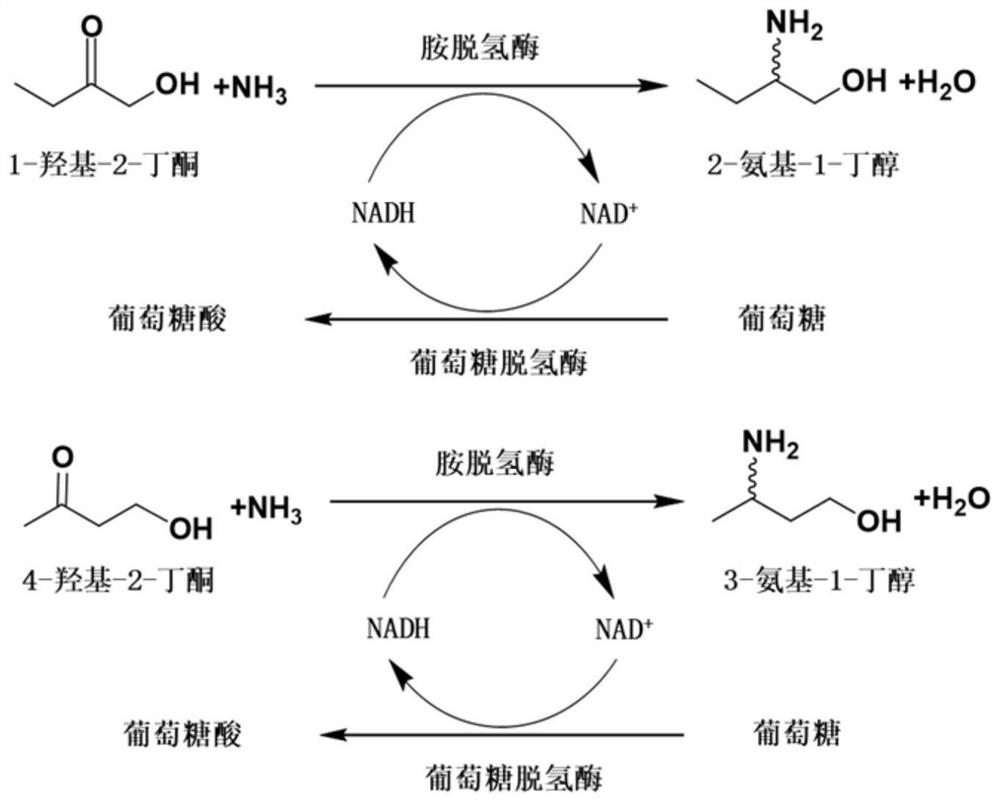
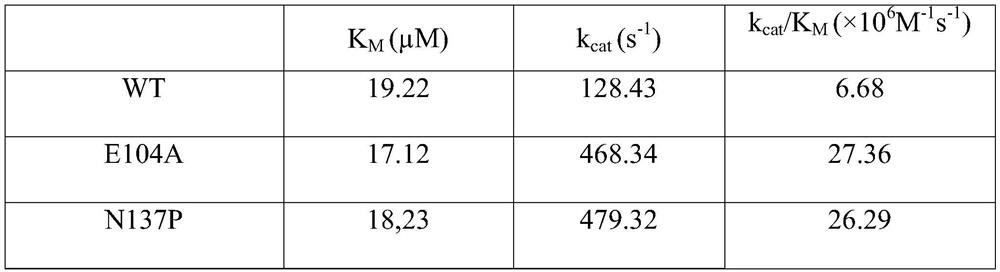
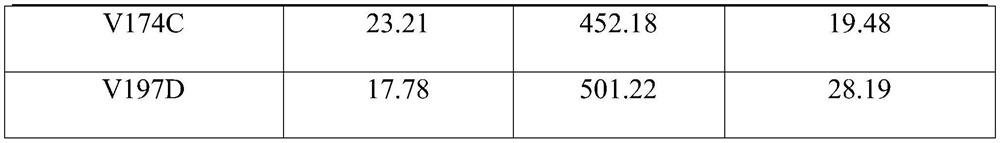
The invention relates to an amine dehydrogenase mutant, an enzyme preparation, a recombinant vector, a recombinant cell, preparation methods therefor and an application of the amine dehydrogenase mutant. The amine dehydrogenase mutant is obtained after a 104 glutamic acid of wild type amine dehydrogenase is mutated into alanine, or obtained after a 137 aspartic acid of the wild type amine dehydrogenase is mutated into proline, or obtained after a 174 valine of the wild type amine dehydrogenase is mutated into cysteine, or obtained after a 197 valine of the wild type amine dehydrogenase is mutated into aspartic acid. The amine dehydrogenase is relatively high in catalysis efficiency.
Owner:SUZHOU INST OF BIOMEDICAL ENG & TECH CHINESE ACADEMY OF SCI
Amine dehydrogenase as well as coding nucleic acid and application thereof
The invention provides amine dehydrogenase as well as coding nucleic acid and application thereof, the amine dehydrogenase is obtained by mutating amino acid dehydrogenase as shown in SEQ ID NO.2, and the mutation comprises at least one mutation in 67th, 113th, 260th and 291th amino acids as shown in SEQ ID NO.2. The amine dehydrogenase disclosed by the invention has relatively high reductive amination activity and high yield, meets the requirement of 99% + stereoselectivity, and has a very wide application prospect.
Owner:HEBEI UNIV OF TECH
A method and kit for stable enzymatic detection of sialic acid
Owner:BEIJING STRONG BIOTECH INC
Amine dehydrogenase and application thereof
ActiveCN110577941AGood choiceHigh catalytic activityOxidoreductasesFermentationAmine dehydrogenaseChiral amine
The invention discloses amine dehydrogenase and application thereof. The amine dehydrogenase is obtained by conducting mutation on amino acid dehydrogenase shown as SEQ ID NO.01, and mutation comprises at least one of the following: G at a 131<st> location is mutated into L or M, N at a 262<nd> location is mutated into V or L, Y at a 285 location is mutated into L or M, and M at a 333<rd> location is mutated into D. A catalytic reaction of the amine dehydrogenase is convenient to operate, the amine dehydrogenase has the advantages of high product optical purity, high yield and the like, equipment is simple, and the amine dehydrogenase has good industrial application prospects in the field of preparation of chiral amine through biological catalysis and high-value development of marine biological resources.
Owner:XIAMEN UNIV
Method for preparing chiral amine through asymmetric reduction under catalysis of marine strain
InactiveCN103224963BImprove responseOptimizationMicroorganism based processesFermentationMicroorganismKetone
The invention provides a method for preparing chiral amine through asymmetric reduction under the catalysis of a marine strain, and relates to chiral amine. The method comprises the following steps: inoculating a strain into a 216L culture medium, culturing, then centrifuging the obtained fermentation liquid to obtain cells, and redissolving and washing with a buffer solution, thus preparing cell sap; and adding ketone used as a substrate into the obtained cell sap, then adding an amino donor and a cosubstrate for cyclic regeneration of coenzyme, and performing asymmetric reduction and amination under the catalysis of the cells, thus obtaining the chiral amine product. The new strain which contains NADH (nicotinamide adenine dinucleotide)-dependent amine dehydrogenase and is different from strains reported before is obtained through the screening of marine microorganisms, and the reaction system and reaction conditions of preparing chiral amine through the asymmetric reduction and amination of ketone under the catalysis of full cells of the strain are optimized. The optical purity of the obtained chiral amine product is up to 90% or above, and the yield is up to 70% or above. The method provided by the invention is convenient to operate, has the advantages of high optical purity of the product, high yield and the like, is simple in equipment and has better industrial application prospects in the field of preparing chiral amine under biological catalysis.
Owner:XIAMEN UNIV
Method for preparing chiral amine from multi-enzyme coupled systems
ActiveCN105112468AAchieve recyclingImprove efficiencyOxidoreductasesFermentationCoupling systemAmine dehydrogenase
The invention discloses a method for preparing chiral amine from multi-enzyme coupled systems and belongs to the technical field of biological catalysis asymmetric conversion. An amine dehydrogenase and glycerol dehydrogenase coupled system, an amine dehydrogenase and formate dehydrogenase coupled system as well as an amine dehydrogenase, glycerol dehydrogenase and formate dehydrogenase coupled system are constructed, and asymmetric preparation of the chiral amine and regeneration of coenzyme nicotinamide adenine dinucleotide are realized. The multi-enzyme coupled systems and the method for preparing the chiral amine from the multi-enzyme coupled systems have advantages that the product conversion rate is high, the reaction condition is mild, operation is simple, the coenzyme can be regenerated and the like, the cost is low, the enzyme utilization rate is high, and the method is suitable for industrial production.
Owner:XIAMEN UNIV
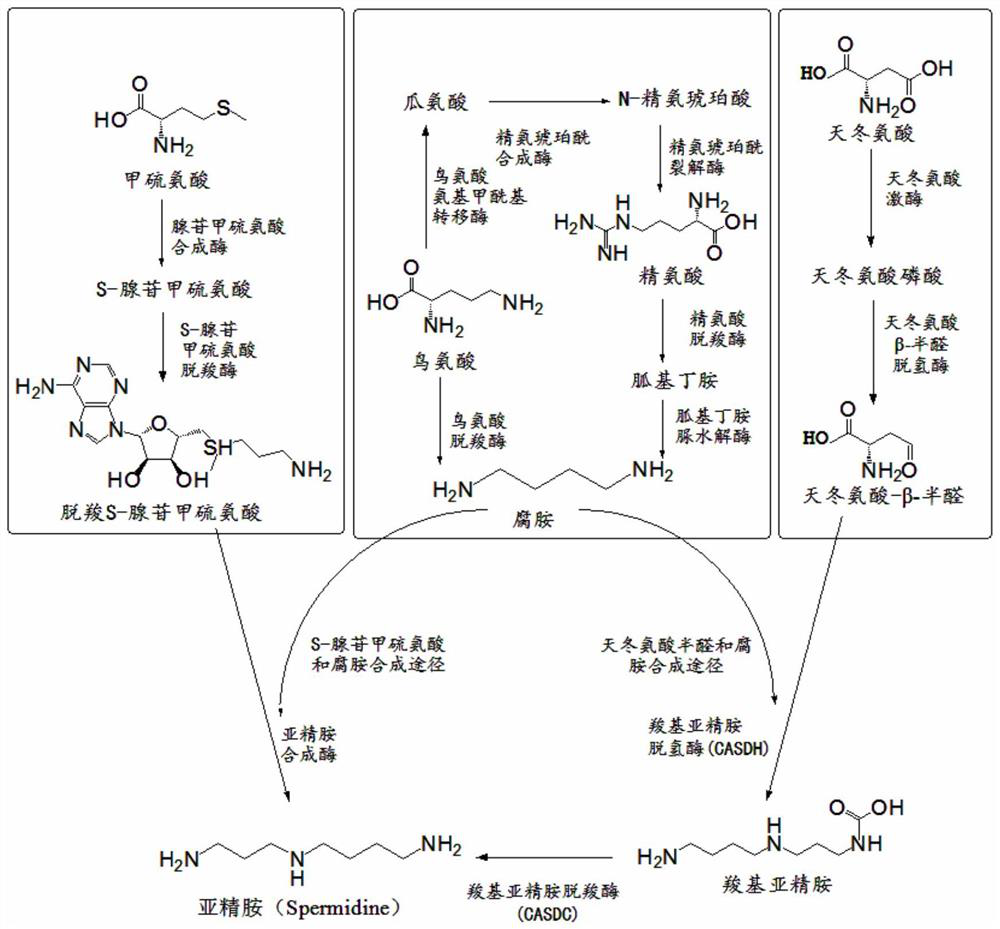
Method for producing spermidine by using cheap substrates and engineering bacterium
ActiveCN112442518ASimple methodRaw materials are easy to getBacteriaTransferasesCarboxyl radicalArginine
The invention discloses a method for producing spermidine by using cheap substrates and engineering bacteria, and belongs to the technical field of bioengineering. The method is characterized in thatrecombinant cells or a combination of recombinant cells expressing gamma-glutamyl kinase, glutamate-5-semialdehyde dehydrogenase, aspartokinase, aspartate-beta-semialdehyde dehydrogenase, amine dehydrogenase, L-2,4-diaminobutanoate decarboxylase, carboxyspermidine dehydrogenase, carboxyspermidine decarboxylase, glucose dehydrogenase, and polyphosphate kinase 2-I are constructed; and aspartic acidand arginine are catalyzed to synthesize the spermidine by utilizing the recombinant cells or the combination of the recombinant cells. According to the method, selected oxidoreductase can efficientlyuse NAD (NADH) as coenzyme, and the product feedback inhibition is avoided in a reaction process; and the method has a good industrial application prospect.
Owner:卓虹超源生物科技(郑州)有限公司
Redox Self-Sufficient Biocatalytic Amination of Alcohols
InactiveUS20170145451A1Elevated atom efficiencyEnvironmental impact factorOxidoreductasesFermentationMicroorganismAlcohol
The present invention relates to a novel biocatalytic method for the production of primary and secondary amines, comprising coupled enzymatic oxidation and reduction processes simultaneously regenerating the required cofactor; making use of two enzymes—i.e. an alcohol dehydrogenase and an amine dehydrogenase—operating simultaneously in a one pot process (i.e. biocatalytic cascade). Furthermore, the overall process is redox neutral, since the hydride generated from the first oxidative step is internally recycled in the second reductive step. The invention also relates to recombinant expression systems and microorganisms procuring the required enzyme activities; and bioreactors for performing such methods.
Owner:BASF AG
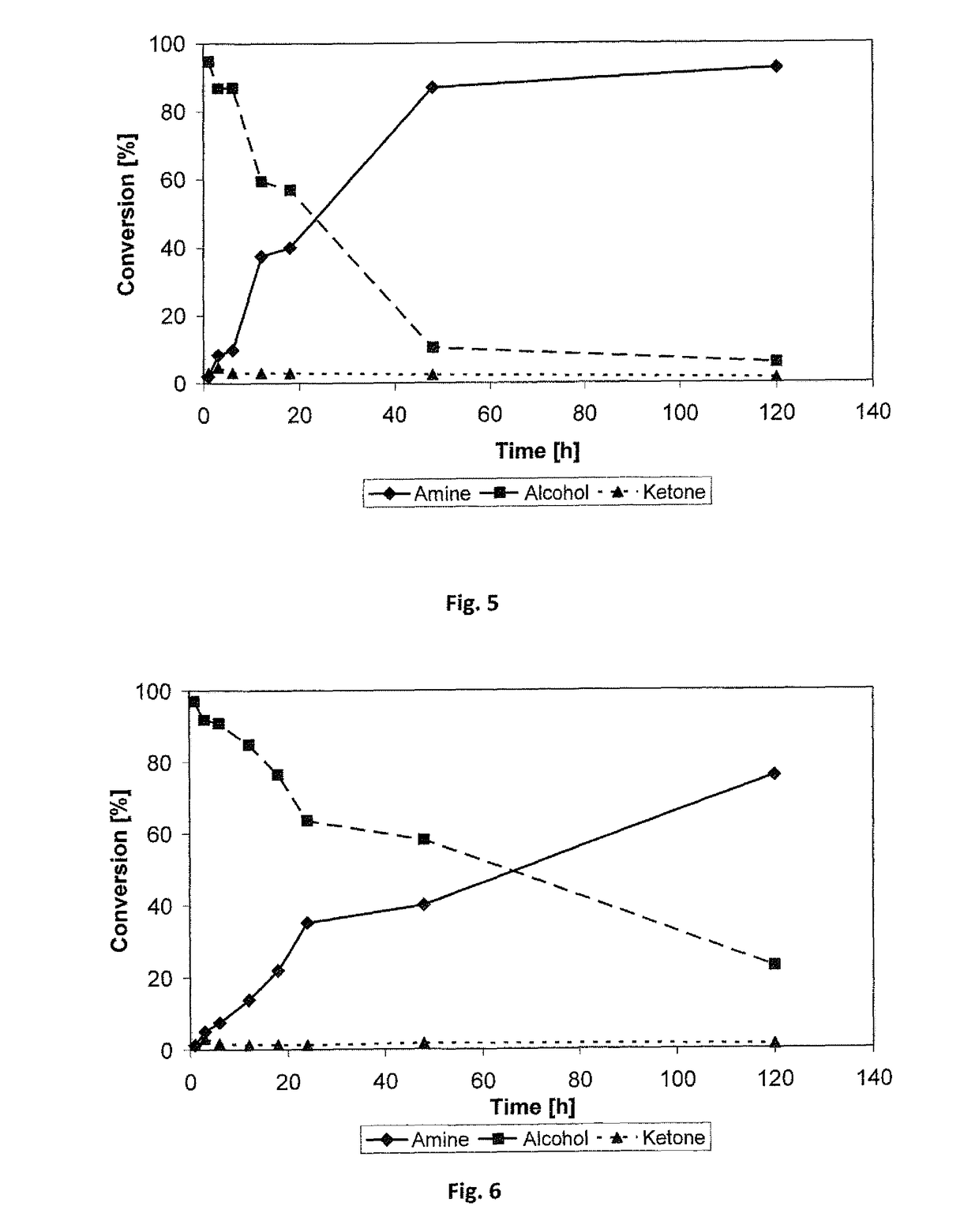
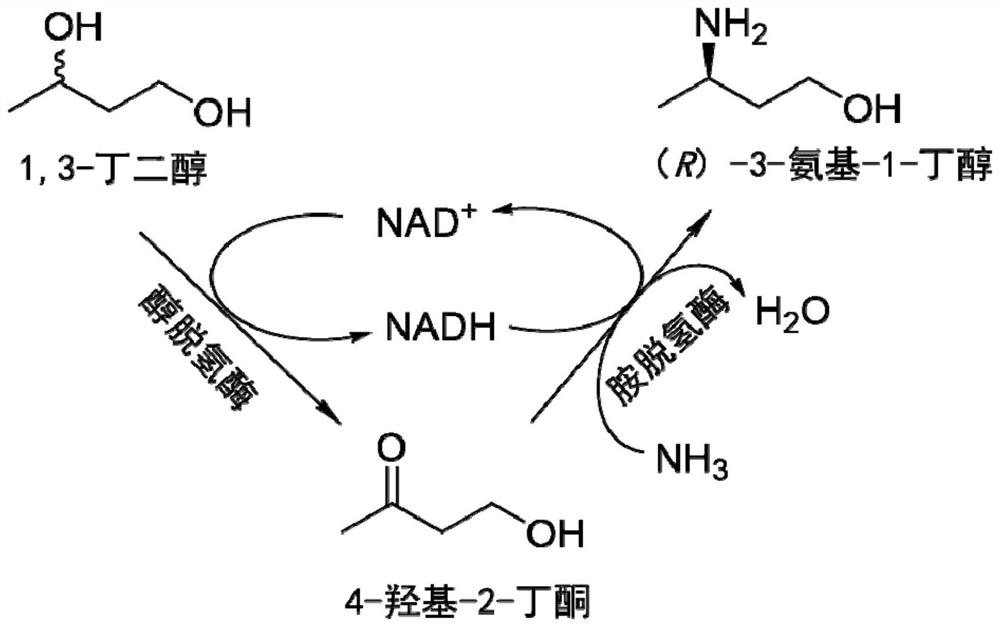
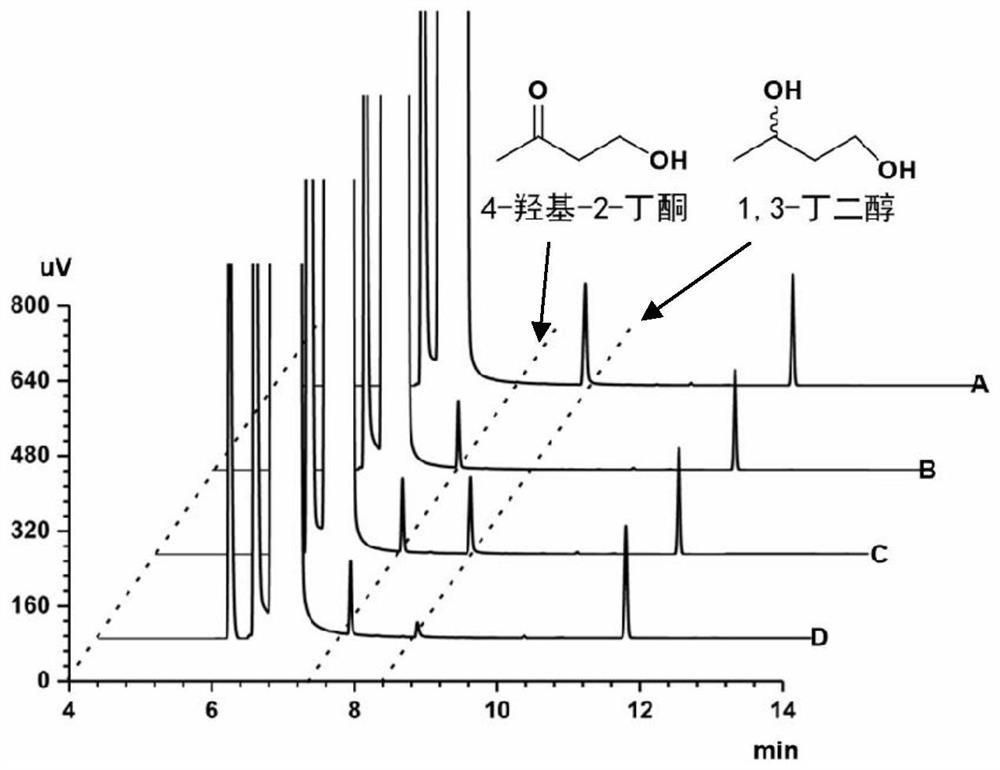
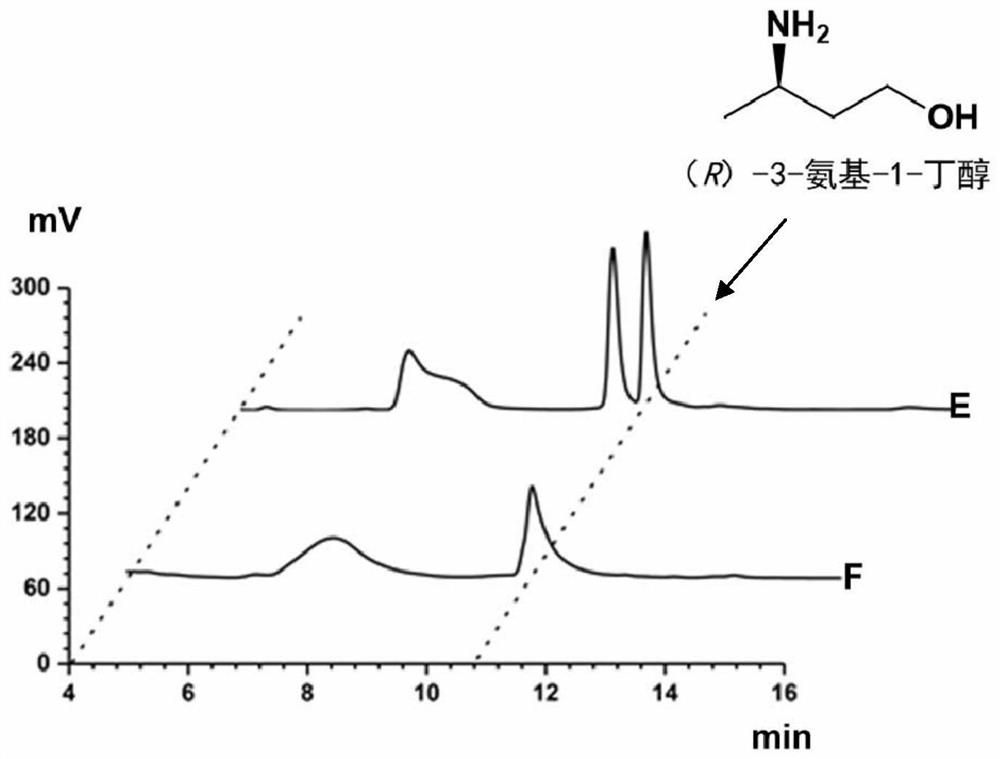
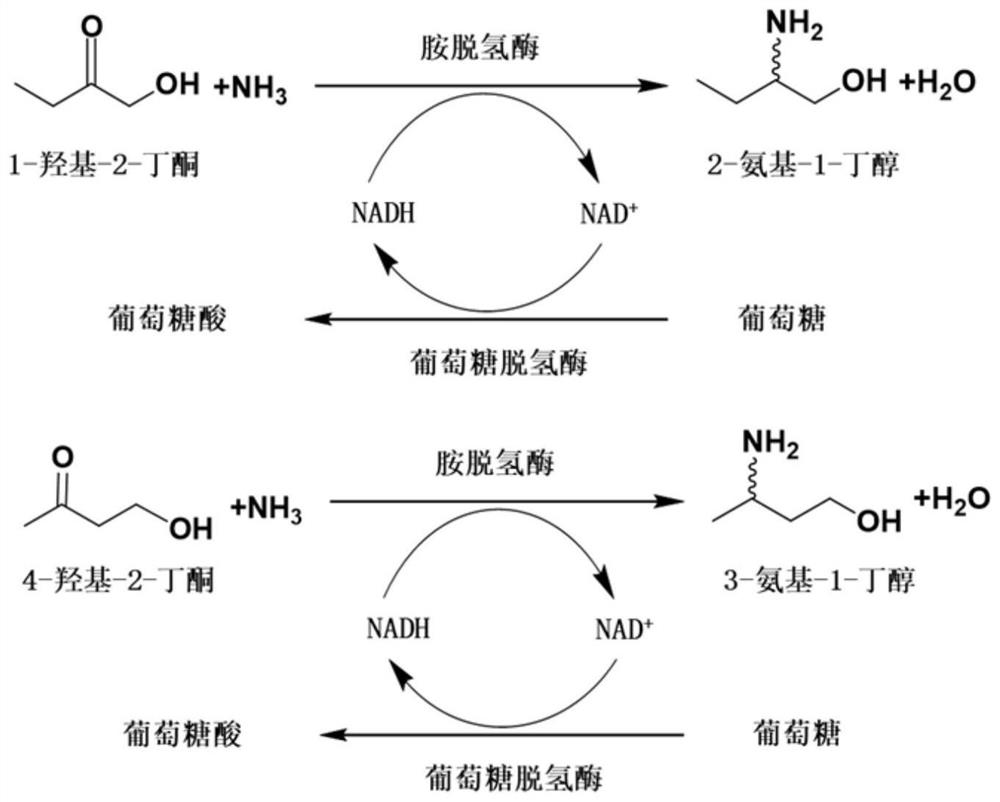
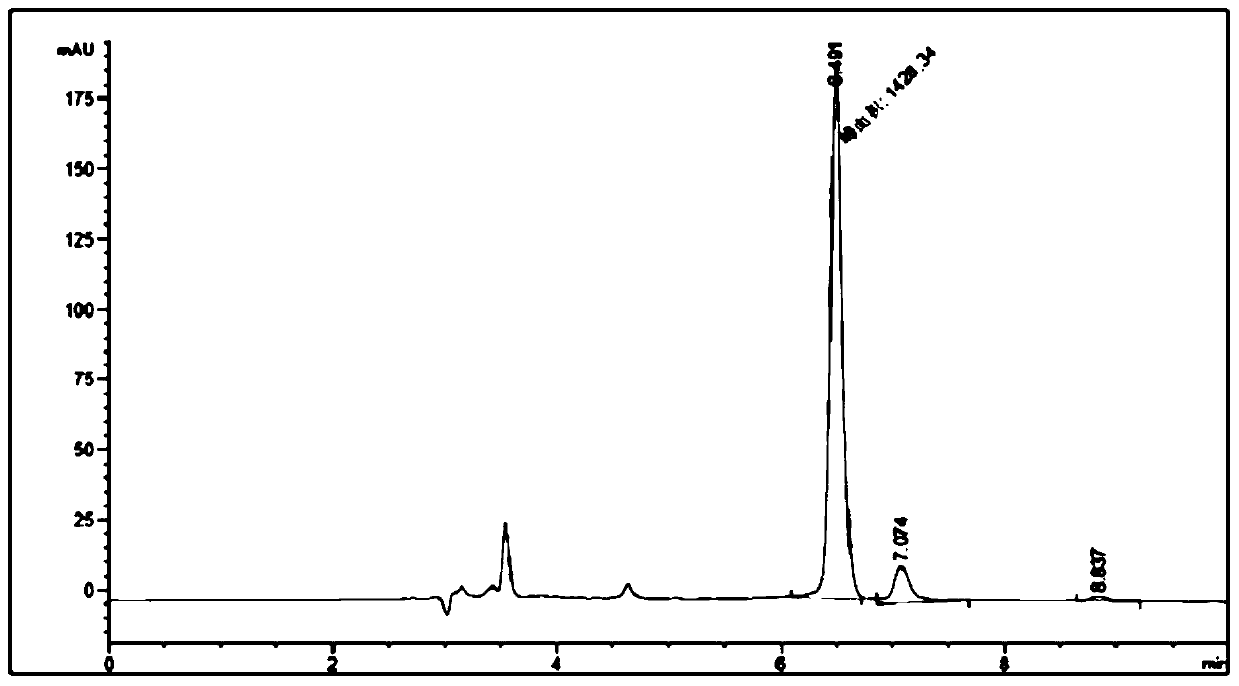
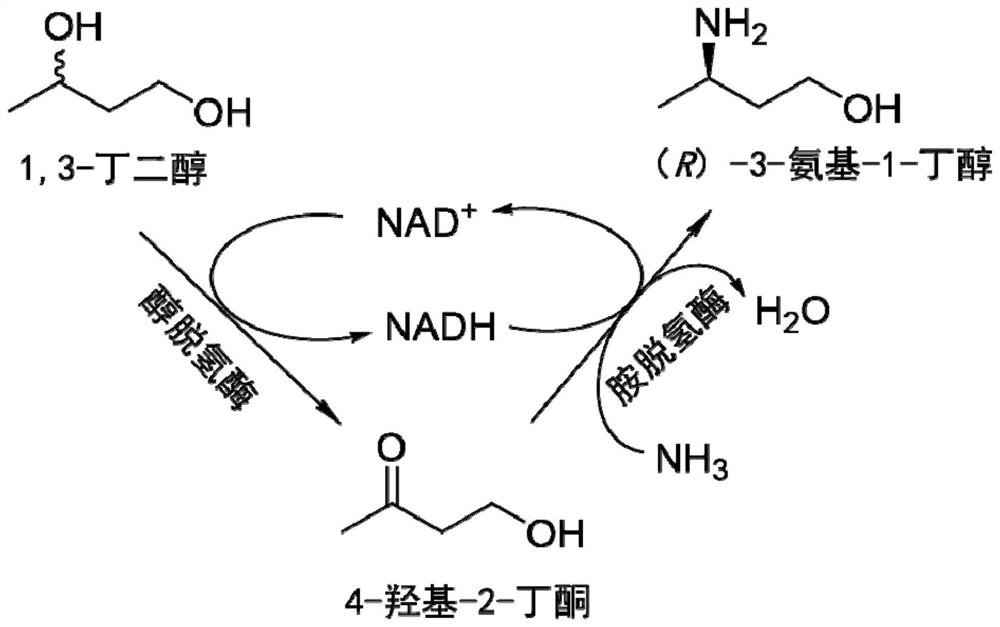
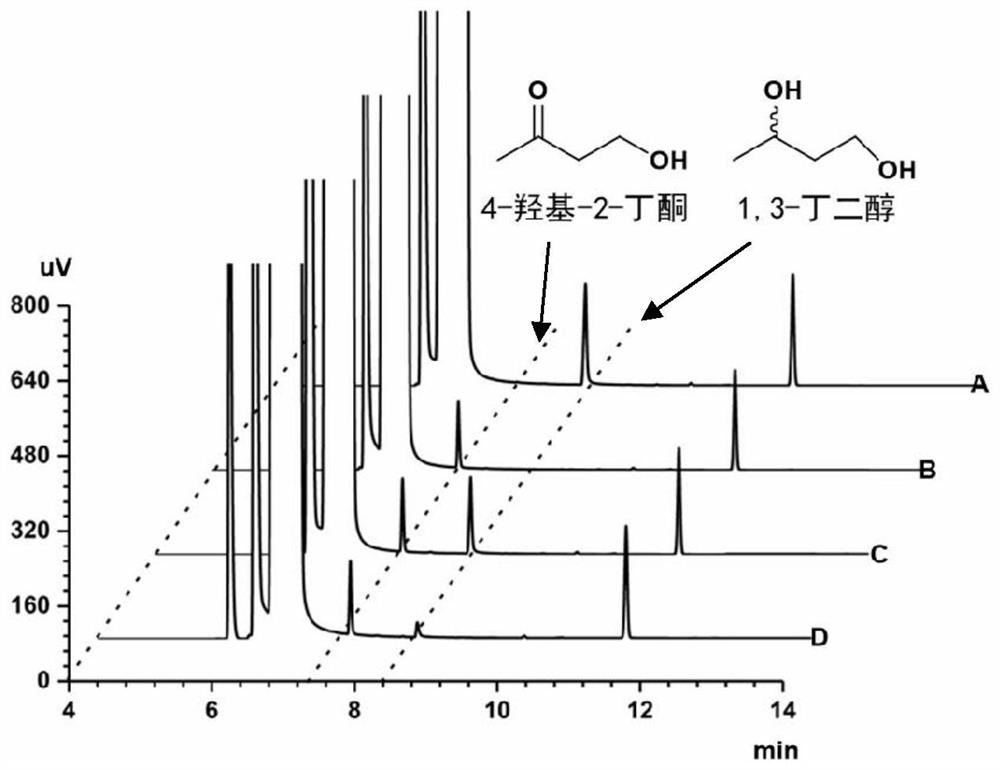
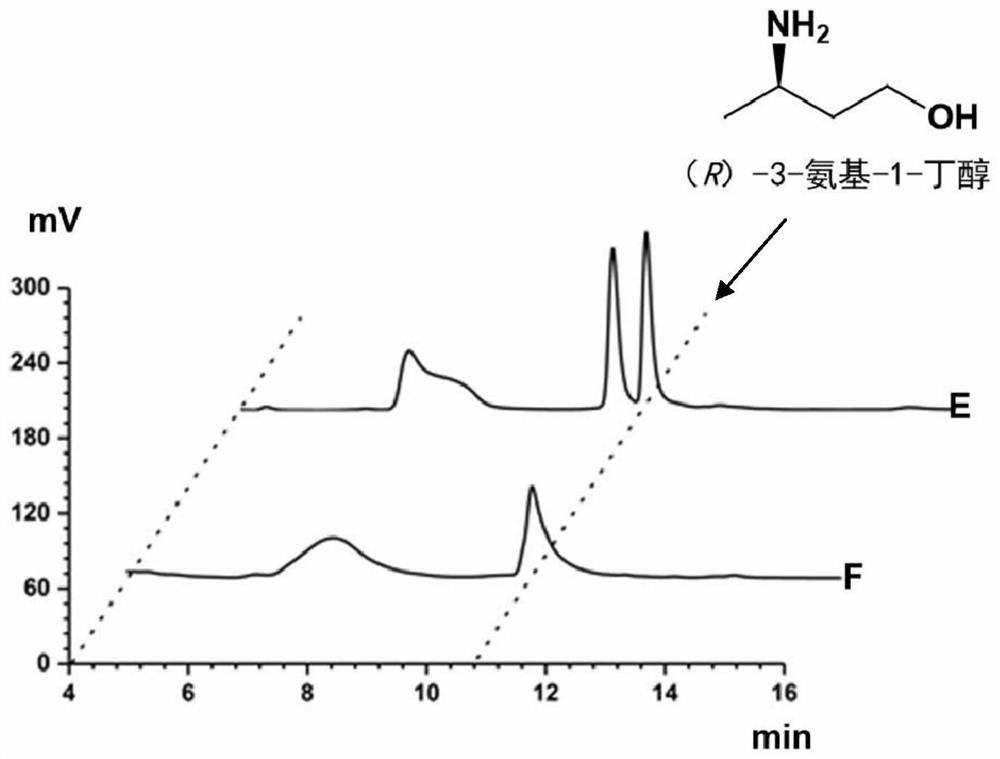
Method for synthesizing (R)-3-amino-1-butanol through double-enzyme cascade catalysis
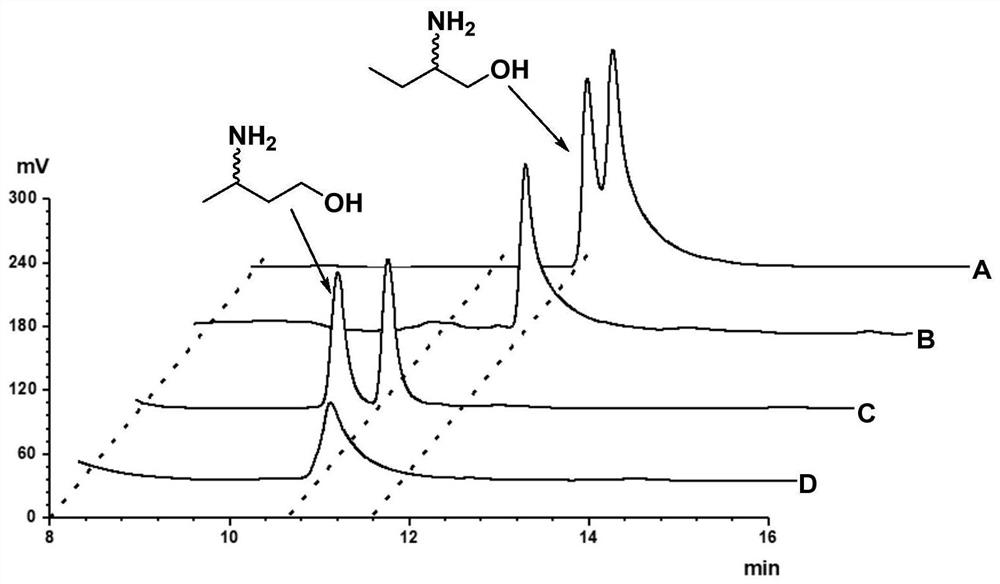
The invention discloses a method for synthesizing (R)-3-amino-1-butanol through double enzyme cascade catalysis. The method comprises the following steps: with 1, 3-butanediol as a substrate, carrying out a catalytic reaction with alcohol dehydrogenase to generate 4-hydroxy-2-butanone; and taking 4-hydroxy-2-butanone as a substrate, and generating chiral (R)-3-amino-1-butanol through a catalytic reaction of amine dehydrogenase or a mutant of the amine dehydrogenase. The invention provides a brand-new green biosynthesis route, and the chiral (R)-3-amino-1-butanol is catalytically synthesized by using cheap 1, 3-butanediol as a raw material through double-enzyme cell co-expression. Meanwhile, the method provided by the invention has a cofactor self-circulation system and has good economic benefits. The method has an important application value.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
Amine dehydrogenase mutant and application thereof in preparation of (S)-5-methyl-2-pyrrolidone
PendingCN114686451AImprove catalytic performanceHigh catalytic activityOxidoreductasesGenetic engineeringPropanoic acidPyrrolidinones
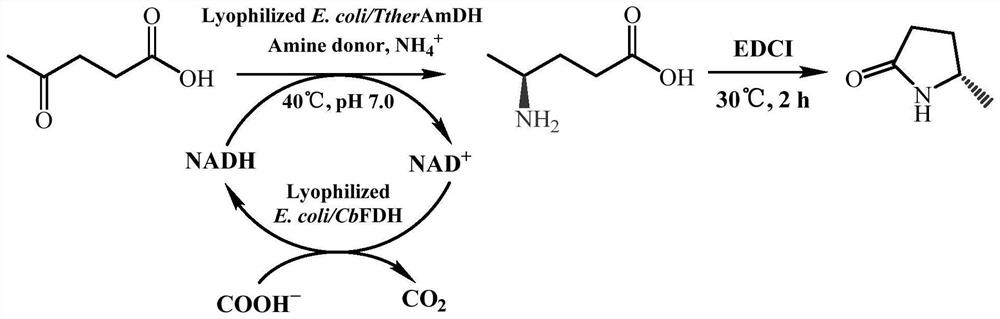
The invention relates to an amine dehydrogenase mutant and an application of the amine dehydrogenase mutant in preparation of (S)-5-methyl-2-pyrrolidone. Specifically, the invention discloses an amine dehydrogenase mutant with improved activity, a coding gene thereof, a recombinant expression vector and a recombinant expression transformant containing the gene sequence, and application of the amine dehydrogenase mutant as a catalyst to catalysis of asymmetric reductive amination of prochiral carbonyl compounds. The invention particularly relates to an application of catalyzing asymmetric reduction of levulinic acid to prepare optically pure 5-methyl-2-pyrrolidone. Compared with the prior art, the method has the advantages of high enzymatic reaction substrate concentration, mild reaction conditions, environment friendliness, high yield, high product optical purity and the like, and has a very good application prospect.
Owner:EAST CHINA UNIV OF SCI & TECH
Amphetamine dehydrogenase mutant and application thereof in chiral amine synthesis
ActiveCN113846069AImprove catalytic performanceHigh catalytic concentrationBacteriaMicroorganism based processesKetoneMutant
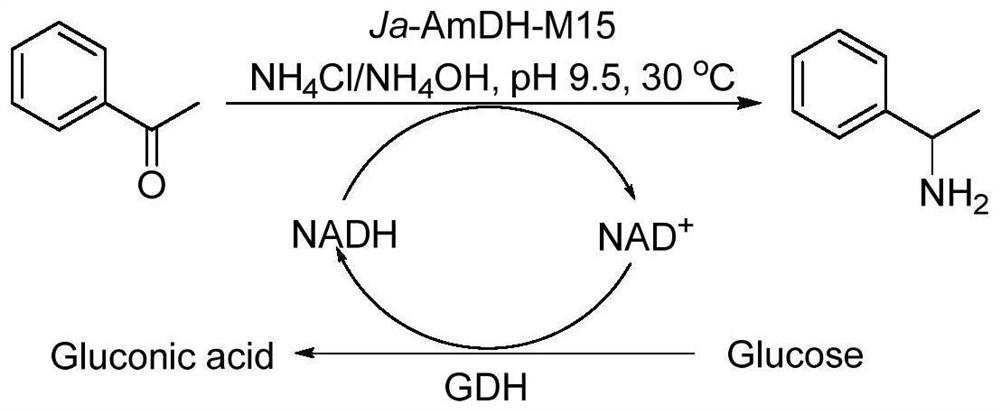
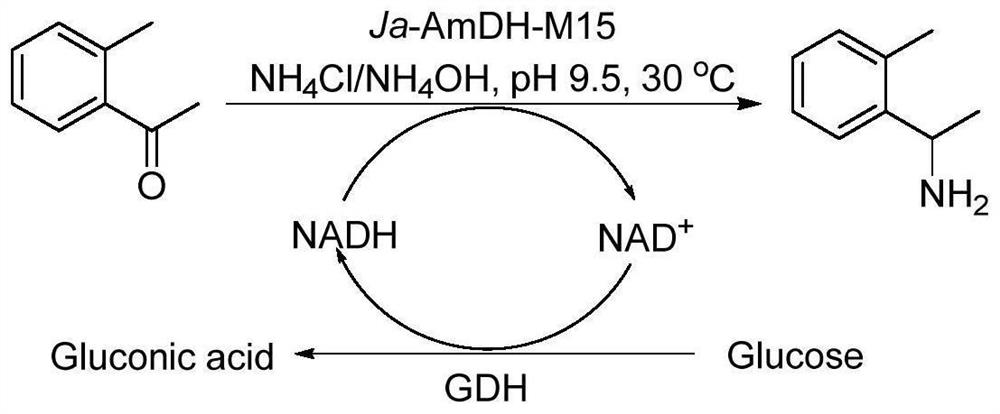
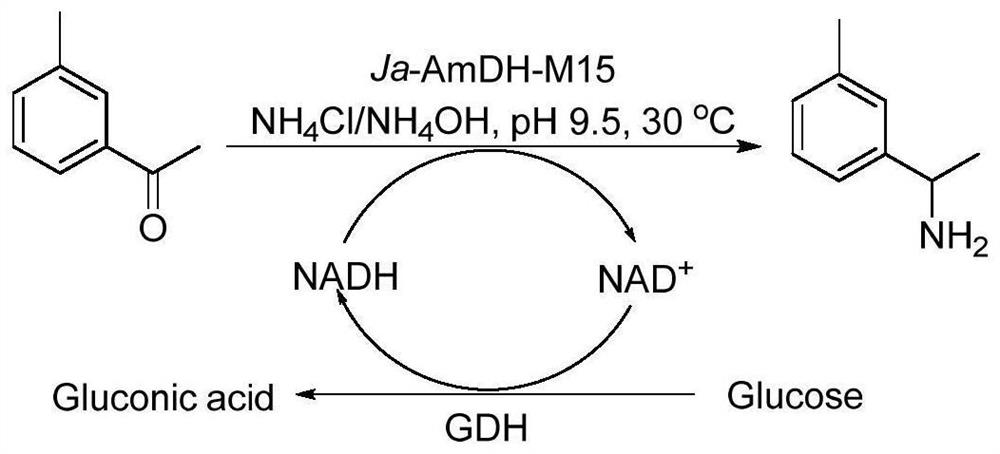
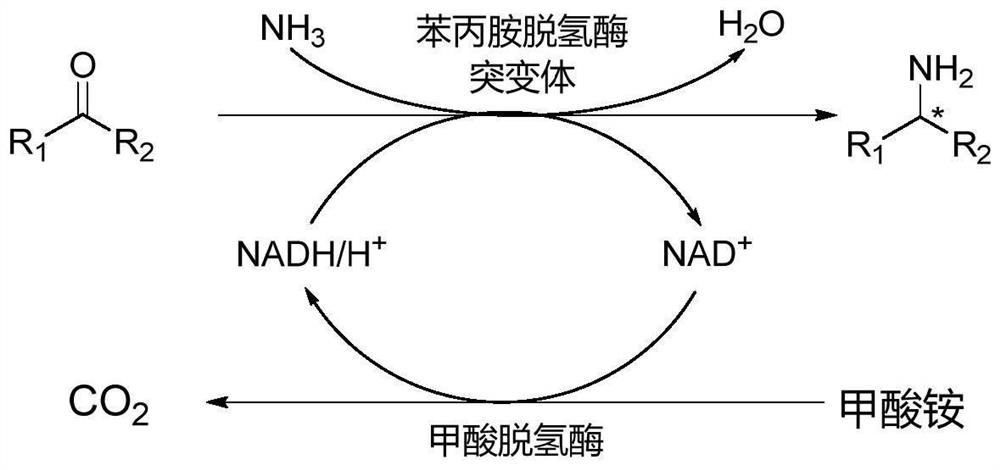
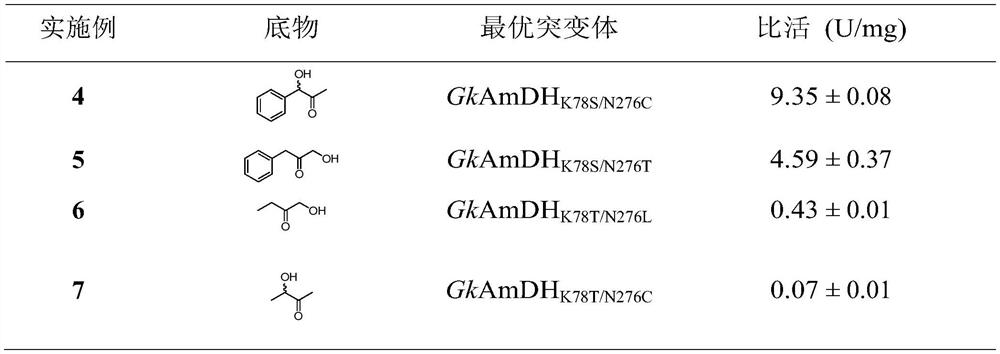
The invention discloses an amphetamine dehydrogenase mutant derived from geobacillus kaustophilus, a coding gene and an amino acid sequence of the amphetamine dehydrogenase mutant, and a method for preparing corresponding chiral amine by catalyzing reductive amination of a large-steric-hindrance carbonyl compound and ammonia by using the amphetamine dehydrogenase mutant. The developed amine dehydrogenase mutant can catalyze carbonyl compounds such as large steric hindrance aromatic ketones, alkyl ketones, heterocyclic ketones and functionalized ketones which are difficult to be reduced and aminated by reported enzymes at present. Compared with other preparation methods, the method for synthesizing the chiral amine by catalyzing asymmetric reductive amination of ketone and ammonia through amine dehydrogenase has the advantages that the used amino donor is cheap ammonia water, relatively expensive organic amine is replaced, a byproduct generated in the reaction is only water. Therefore, the method has the advantages of high atom economy, high optical purity of the product, mild reaction conditions, environmental friendliness, simple post-treatment of the product and the like, and has a good application prospect in synthesis of the chiral primary amine.
Owner:EAST CHINA UNIV OF SCI & TECH
Amine dehydrogenase mutant and application thereof in synthesis of chiral amine alcohol compound
ActiveCN112852894AHigh yieldHigh optical purityBacteriaMicroorganism based processesAlcoholChemical compound
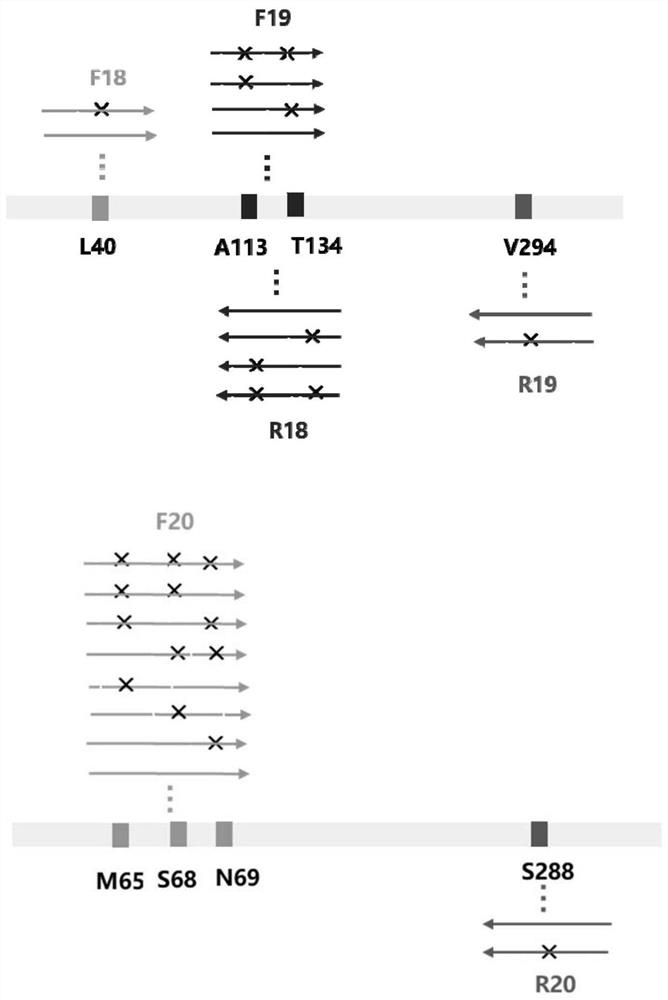
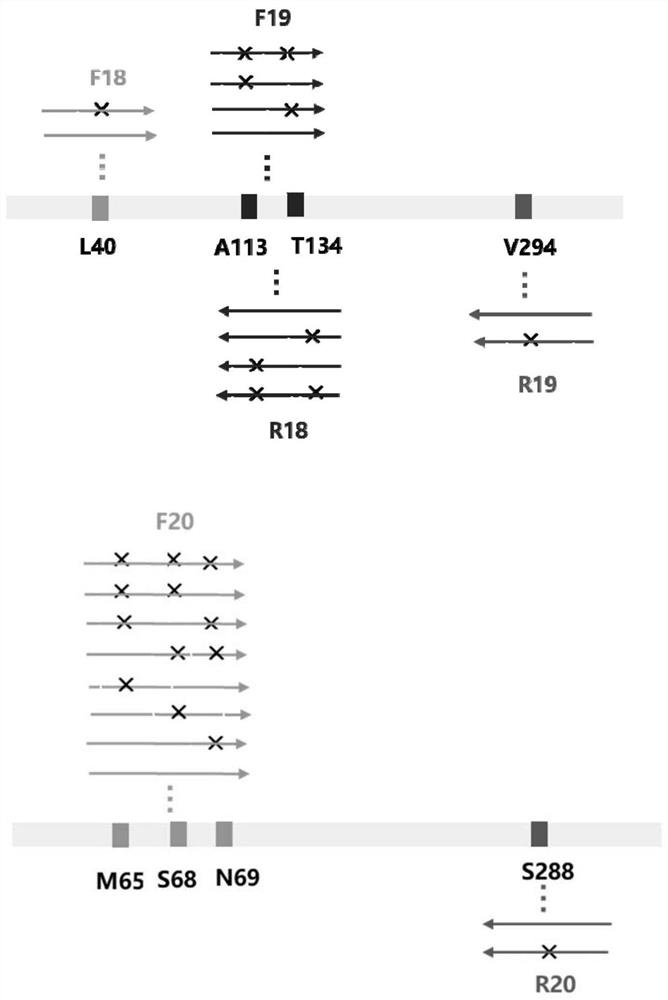
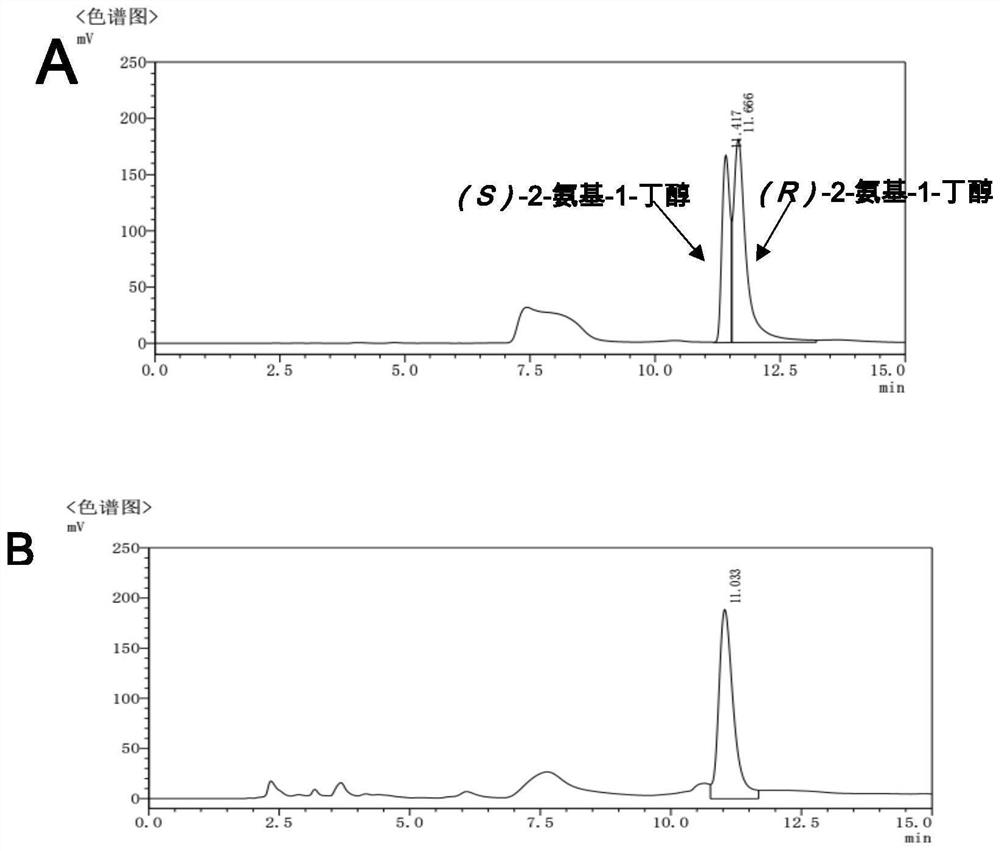
The invention discloses an amine dehydrogenase mutant and an application thereof in synthesis of a chiral amine alcohol compound. The amine dehydrogenase mutant disclosed by the invention is a protein obtained by mutating the 40th site, the 61th site, the 68th site, the 69th site, the 111st site, the 114th site, the 116th site, the 150th site, the 187th site, the 239th site, the 288 site, the 291 site, the 292 site, the 295th site, the 297th site and / or the 294th site of the amine dehydrogenase (SpAmDH) derived from Sporosarcina psychrophila. The amine dehydrogenase mutant can catalyze hydroxy ketone substrates to synthesize chiral amine alcohol compounds represented by 1-hydroxy-2-butanone and 4-hydroxy-2-butanone, is convenient to operate, has the advantages of high optical purity of products and the like, has stereoselectivity up to 99%, and has a better industrial application prospect in preparation of the chiral amine alcohol compounds through biological catalysis.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
Marine bacterial strain and method for preparing chiral amine from catalyzing of amine dehydrogenase of marine bacterial strain
InactiveCN105567756AHigh catalytic activityImprove catalytic selectivityMicroorganism based processesFermentationSynthesis methodsBacterial strain
The invention discloses a marine bacterial strain and a method for preparing chiral amine from catalyzing of amine dehydrogenase of the marine bacterial strain and belongs to the technical field of asymmetric synthesis chiral compounds obtained through a biological method. Psedomonas balearica from the sea is obtained through screening, and under the condition that ketone serving as a substrate, an amino donor and an auxiliary substrate are added, the chiral amine is prepared by the utilization of asymmetric reductive amination of microorganism and crude enzyme catalyzed ketone of the bacterial strain. A reaction system and reaction conditions of asymmetric reductive amination of microorganism catalyzing and crude enzyme catalyzing of the strain are determined. The method has good industrial application prospects in the field of preparation of chiral amine through biological catalysis and has important meaning for research on development of marine novel biocatalyst and a green synthesis method for chiral amine in the future.
Owner:XIAMEN UNIV
Method for preparing chiral amine through co-immobilization cascade reaction of alcohol dehydrogenase and amine dehydrogenase mediated by silicon dioxide binding peptide
PendingCN114369593AImprove catalytic performanceEasy to usePeptidesOxidoreductasesHigh concentrationPtru catalyst
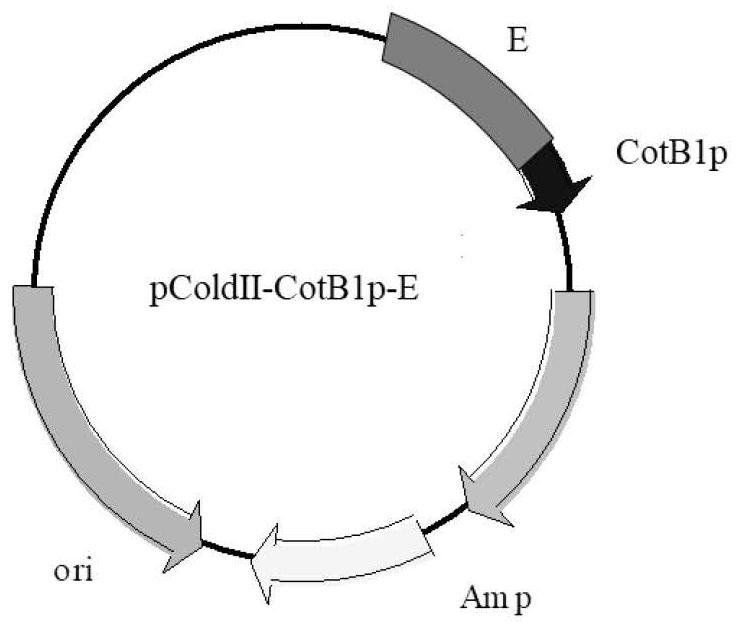
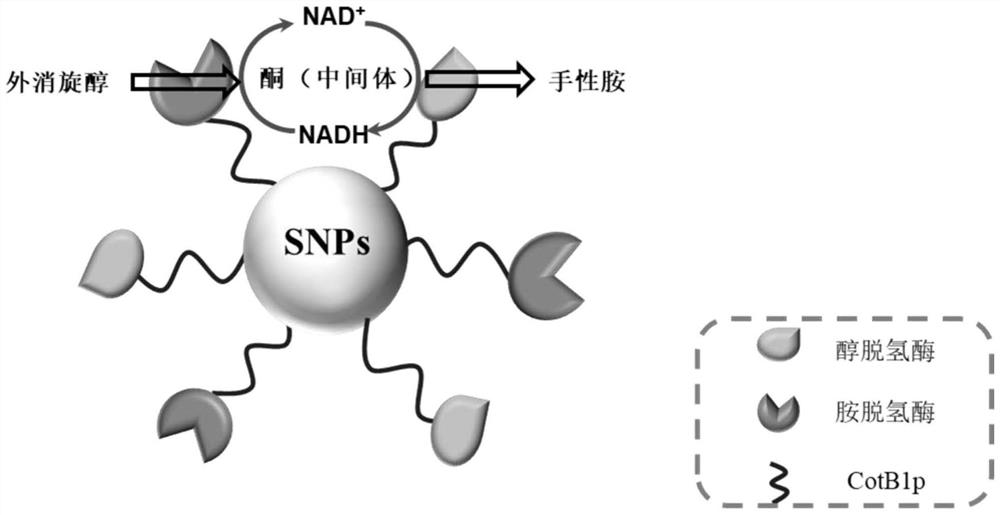
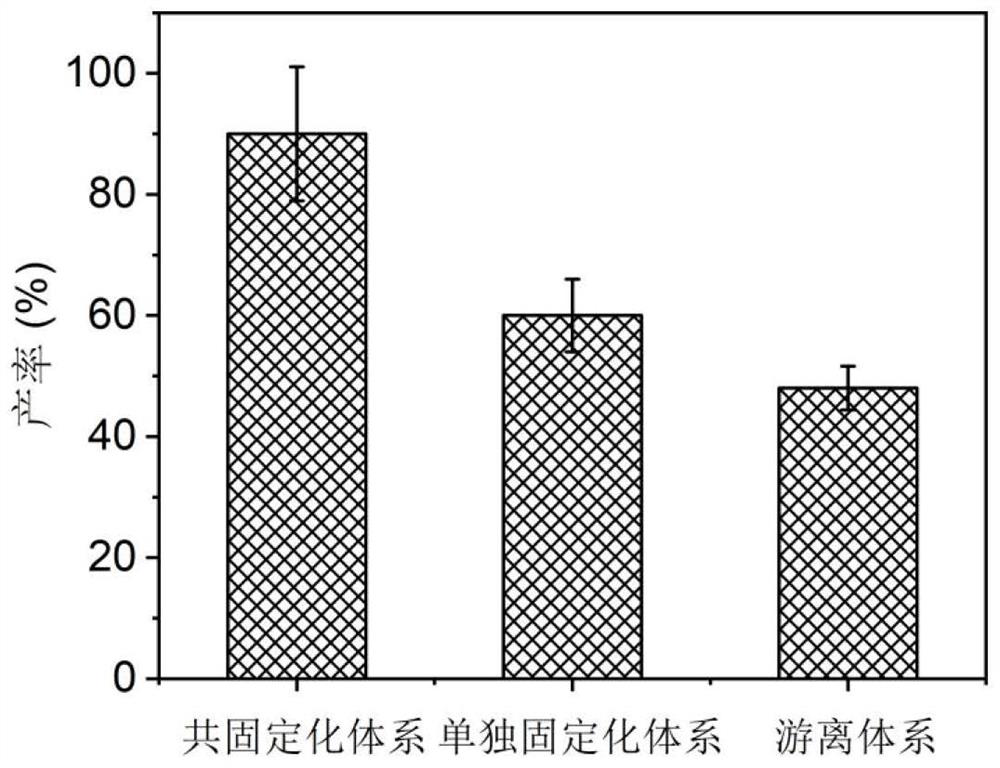
The invention relates to a method for preparing chiral amine through co-immobilization cascade reaction of alcohol dehydrogenase and amine dehydrogenase mediated by silicon dioxide binding peptide. Respectively inserting the genes of the silicon dioxide binding peptide into N tail ends of gene sequences of mediated alcohol dehydrogenase and amine dehydrogenase by adopting an overlapping extension PCR (Polymerase Chain Reaction) technology, and constructing a gene engineering strain; purifying and separating the recombinant protein by using a nickel ion metal chelating affinity chromatographic column and a high-concentration imidazole buffer solution; the method comprises the following steps: by taking silicon dioxide nanoparticles as a carrier of an immobilized enzyme, co-anchoring a recombinant protein on the surface of SNPs to obtain an immobilized enzyme CotB1p-ADHamp; cotB1p-AmDH (at) SNPs (single nucleotide polymorphism); and placing the obtained immobilized enzyme in an NH4Cl buffer solution containing NAD < + > and racemic alcohol for reaction to prepare the chiral amine. The prepared double-enzyme co-immobilized catalyst shows relatively high enzyme loading capacity and improved catalytic activity and stability, and has relatively great potential in the aspect of chiral amine production in industrial application.
Owner:TIANJIN UNIV
Amine Dehydrogenase Mutants and Their Applications in the Synthesis of Chiral Amino Alcohols
ActiveCN112852894BHigh yieldHigh optical purityBacteriaMicroorganism based processesSporosarcina psychrophilaChemical compound
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
Amine dehydrogenase mutant, enzyme preparation, recombinant vector, recombinant cell and its preparation method and application
The invention relates to an amine dehydrogenase mutant, an enzyme preparation, a recombinant vector, a recombinant cell, preparation methods therefor and an application of the amine dehydrogenase mutant. The amine dehydrogenase mutant is obtained after a 104 glutamic acid of wild type amine dehydrogenase is mutated into alanine, or obtained after a 137 aspartic acid of the wild type amine dehydrogenase is mutated into proline, or obtained after a 174 valine of the wild type amine dehydrogenase is mutated into cysteine, or obtained after a 197 valine of the wild type amine dehydrogenase is mutated into aspartic acid. The amine dehydrogenase is relatively high in catalysis efficiency.
Owner:SUZHOU INST OF BIOMEDICAL ENG & TECH CHINESE ACADEMY OF SCI
A mutant of amine dehydrogenase and its application in the synthesis of chiral amines and aminoalcohols
ActiveCN110628739BImprove catalytic performanceHigh optical purityBacteriaMicroorganism based processesPhenylalanine dehydrogenaseAmphetamine use
The invention relates to an amine dehydrogenase mutant and its application in the synthesis of chiral amine and aminoalcohol. Specifically, the present invention discloses an amphetamine dehydrogenase mutant and its corresponding amino acid sequence obtained by molecular engineering of Geobacillus kaustophilus phenylalanine dehydrogenase, and the amphetamine The use of dehydrogenase mutants in the synthesis of chiral amines and chiral amino alcohols by asymmetric reductive amination of latent chiral carbonyl compounds. Compared with the synthesis methods of other chiral amines and chiral amino alcohols, the ammonia donor used in the present invention is cheap ammonia water, the formed by-product is only clean water, and the optical purity of the products is >99%, so it represents A biosynthesis method with mild reaction, high stereoselectivity, and environmental protection has been developed, which has a good application prospect in the actual production of chiral amines and chiral amino alcohols.
Owner:EAST CHINA UNIV OF SCI & TECH +1
A kind of synthetic method of chiral aminoalcohol compound
ActiveCN112779232BHigh optical purityHigh yieldOxidoreductasesFermentationBiological materialsGlycol synthesis
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
A transformation strain and method for catalytically synthesizing miglitol intermediates
ActiveCN110016455BIncreased dehydrogenase activityIncrease vitalityBacteriaMicroorganism based processesMiglitolGluconobacter oxydans
The invention discloses a transformation strain and a method for catalyzing the synthesis of miglitol intermediates. The transformation strain is obtained from the starting strain ZJB16009 by transforming the recombinant expression plasmid pBBR-sldAB, and then screening, named Gluconobacter oxidans ( Gluconobacter oxydans), strain number pBBR‑sldAB, preservation number CCTCC No.M 2019033, its N‑hydroxyethylglucosamine dehydrogenase activity is greatly improved compared with the starting strain ZJB16009, and the space-time yield is about that of the starting strain ZJB16009 2 times, it shows that the Gluconobacter oxydans pBBR-sldAB of the present invention has higher potential application value in the catalytic synthesis of the key intermediate 6NSL of miglitol.
Owner:ZHEJIANG UNIV OF TECH +1
A method for preparing chiral amines catalyzed by a marine strain and its amine dehydrogenase
InactiveCN105567756BHigh catalytic activityImprove catalytic selectivityMicroorganism based processesFermentationMicroorganismSynthesis methods
Owner:XIAMEN UNIV
Method for synthesizing (r)-3-amino-1-butanol by double-enzyme cascade catalysis
ActiveCN112852895BHigh optical purityEasy to operateOxidoreductasesFermentationButanediolAmino radical
The invention discloses a method for synthesizing (R)-3-amino-1-butanol through double-enzyme cascade catalysis. The method comprises the steps of: using 1,3-butanediol as a substrate, generating 4-hydroxy-2-butanone through an alcohol dehydrogenase catalyzed reaction; using 4-hydroxy-2-butanone as a substrate, Dehydrogenase or its mutants catalyze the reaction to generate chiral (R)‑3‑amino‑1‑butanol. The invention provides a brand-new green biosynthesis route, using cheap 1,3-butanediol as a raw material, and catalyzing the synthesis of chiral (R)-3-amino-1-butanol through double-enzyme cell co-expression. At the same time, the method provided by the invention has a cofactor self-circulation system and has good economic benefits. The invention has important application value.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
A kind of polyethyleneimine-titanium oxide embedding amine dehydrogenase and its preparation method
ActiveCN104404026BReduce leak rateGood leak rateOn/in organic carrierOn/in inorganic carrierTitanium nitrideTitanium oxide
The invention relates to polyethylene imine-titanium oxide embedded with amine dehydrogenase and a preparation method of the polyethylene imine-titanium oxide, and belongs to the technical field of immobilized enzymes. A polyethylene imine (PEI)-titanium oxide immobilized particle embedded with the amine dehydrogenase has a grain size of 100-600 nm, wherein polyethylene imine has catalyzing and templating double effects in the immobilization process, namely the PEI promotes the formation of titanium oxide through an electrostatic and hydrogen bonding effect between an amino group in a molecule and a titanium precursor molecule; through a polyethylene imine structure, a template can also be induced, and the formation of a titanium oxide particle are regulated and controlled. The preparation method of the polyethylene imine-titanium oxide comprises the following steps: configuring the PEI (straight chain and branched chain) as well as mixed liquid containing the amine dehydrogenase; configuring a precursor II-dihydroxybis (2-hydroxypropanoato) titante diammonium (Ti-BALDH) solution of titanium; dropwise adding the precursor solution of the titanium into the PEI and the mixed liquid containing the amine dehydrogenase, stirring and generating the PEI-titanium oxide immobilized particle embedded with the amine dehydrogenase. The polyethylene imine-titanium oxide and the preparation method thereof have the advantages that the preparation conditions are mild, the process is simple and is easy to implement, the obtained immobilized particle embedded with the amine dehydrogenase has strong immobilization capacity and good reuse stability, and the temperature stability of the amine dehydrogenase can be obviously improved.
Owner:XIAMEN UNIV
A kind of method for preparing chiral amine by multi-enzyme coupling system
ActiveCN105112468BImprove the efficiency of preparing chiral aminesRealize the regeneration cycleOxidoreductasesFermentationCoupling systemAmine dehydrogenase
The invention discloses a method for preparing chiral amine from multi-enzyme coupled systems and belongs to the technical field of biological catalysis asymmetric conversion. An amine dehydrogenase and glycerol dehydrogenase coupled system, an amine dehydrogenase and formate dehydrogenase coupled system as well as an amine dehydrogenase, glycerol dehydrogenase and formate dehydrogenase coupled system are constructed, and asymmetric preparation of the chiral amine and regeneration of coenzyme nicotinamide adenine dinucleotide are realized. The multi-enzyme coupled systems and the method for preparing the chiral amine from the multi-enzyme coupled systems have advantages that the product conversion rate is high, the reaction condition is mild, operation is simple, the coenzyme can be regenerated and the like, the cost is low, the enzyme utilization rate is high, and the method is suitable for industrial production.
Owner:XIAMEN UNIV
Construction and application of amine dehydrogenase mutants with improved thermostability and genetically engineered bacteria
ActiveCN110846291BImprove thermal stabilityImprove stabilityOxidoreductasesFermentationEngineered geneticMutant
The invention belongs to the technical field of biology, in particular to an amine dehydrogenase mutant with improved thermal stability, a construction method and an application of genetic engineeringbacteria. The amine dehydrogenase mutant comprises 4 single-site mutants and 11 combined mutants, and compared with wild-type amine dehydrogenase, the single-site mutants and the combined mutants have longer half-life period at 42 DEG C; in particular, the combined mutants show a superposition effect of thermal stability of the single-site mutants, and the half-life period is about 5 times that of the wild-type amine dehydrogenase. The amine dehydrogenase mutant obtained by the construction method has better thermal stability, shows excellent stereoselectivity, regioselectivity and catalyticactivity when being catalyzed for synthesis of chiral amine at higher temperature, and has a better application prospect.
Owner:SUZHOU INST OF BIOMEDICAL ENG & TECH CHINESE ACADEMY OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com