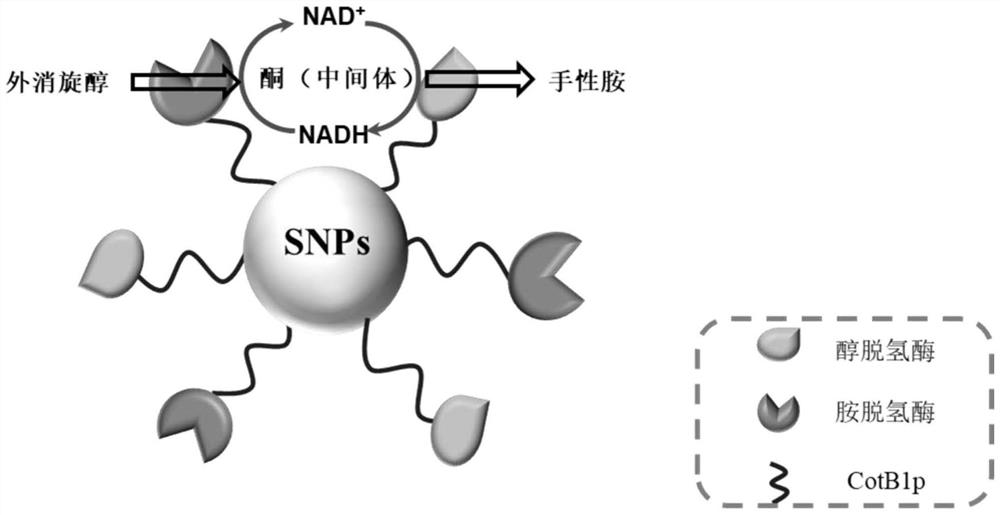
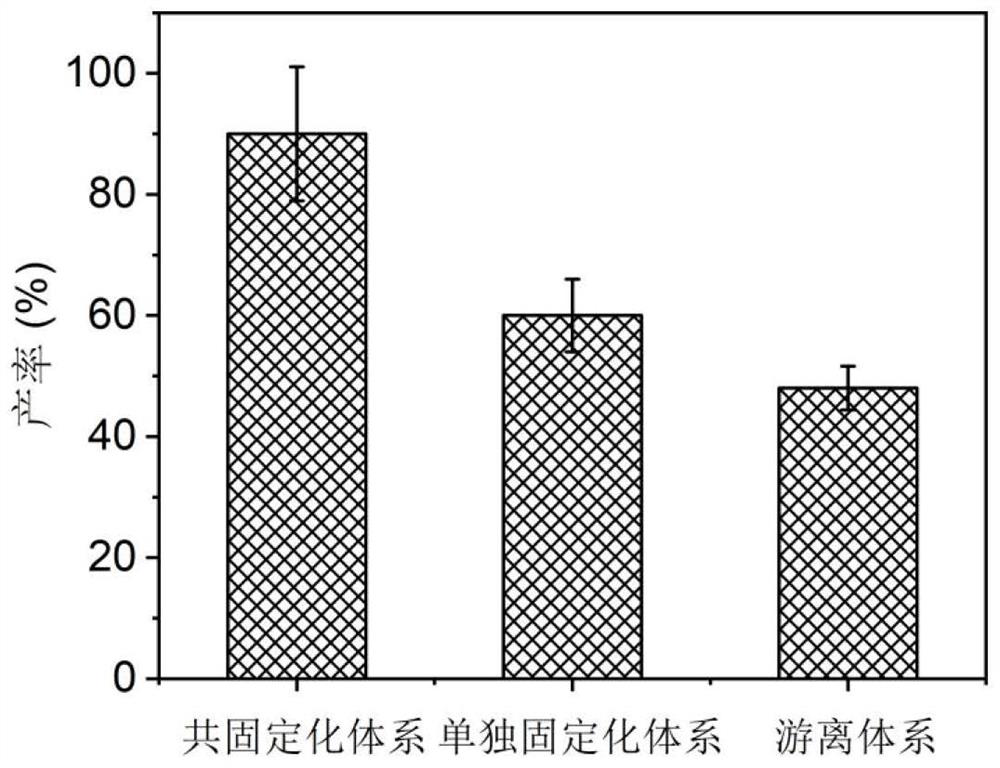
Method for preparing chiral amine through co-immobilization cascade reaction of alcohol dehydrogenase and amine dehydrogenase mediated by silicon dioxide binding peptide
A technology of silica and alcohol dehydrogenase, which is applied in the fields of biochemical equipment and methods, oxidoreductases, and botanical equipment and methods, and can solve the problem that single-enzyme catalysis cannot meet production needs, small specific surface area, and is not conducive to immobilization. The contact between enzyme and substrate molecule, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
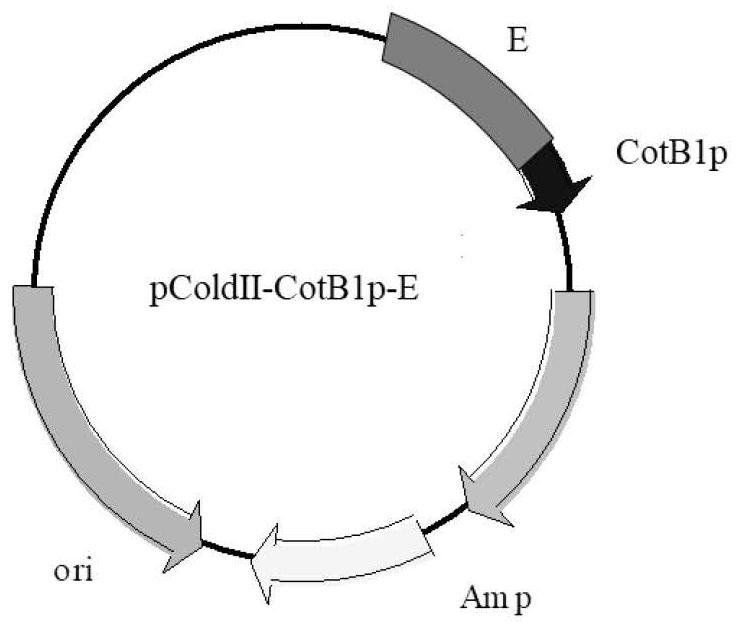
[0067] 1) Pass the gene (SEQ ID NO.1) of CotB1p through (G 4 S) 2 The connecting peptide was fused with the N-terminus of the ADH (SEQ ID NO.2) and AmDH (SEQ ID NO.3) genes respectively to obtain CotB1p-ADH and CotB1p-ADH with gene sequences of SEQ ID NO.4 and SEQ ID NO.5 respectively AmDH, and then subcloning the fused gene into the restriction site between NdeI and XhoI of plasmid pColdII (SEQ ID NO.6) to form recombinant plasmids (pColdII-CotB1p-ADH, pColdII-CotB1p-AmDH) , which was verified by sequencing, transformed into Escherichia coli shuffle T7-B competent cells, and obtained genetically engineered strains;
[0068] 2) The genetically engineered strains are cultured in two stages, first grown to OD at 37°C 600nm= 1.0, then add 0.1M IPTG at 16°C to induce recombinant protein expression for 24 hours, collect the expression strain by centrifugation (4000g, 30min) after induction, and store it at -20°C for later use;
[0069] 3) Resuspend the above obtained strain in l...
Embodiment 2
[0075] 1) Pass the gene (SEQ ID NO.1) of CotB1p through (G 4 S) 2 The connecting peptide was fused with the N-terminus of the ADH (SEQ ID NO.2) and AmDH (SEQ ID NO.3) genes respectively to obtain CotB1p-ADH and CotB1p-ADH with gene sequences of SEQ ID NO.4 and SEQ ID NO.5 respectively AmDH, and then subcloning the fused gene into the restriction site between NdeI and XhoI of plasmid pColdII (SEQ ID NO.6) to form recombinant plasmids (pColdII-CotB1p-ADH, pColdII-CotB1p-AmDH) , which was verified by sequencing, transformed into Escherichia coli shuffle T7-B competent cells, and obtained genetically engineered strains;
[0076] 2) The genetically engineered strains are cultured in two stages, first grown to OD at 37°C 600nm = 1.0, then add 0.1M IPTG at 16°C to induce the expression of CotB1p-ADH for 24 hours, and collect the expression strain by centrifugation (4000g, 30min) after the induction, and store it at -20°C for later use;
[0077] 3) Resuspend the above obtained stra...
Embodiment 3
[0083]1) Pass the gene (SEQ ID NO.1) of CotB1p through (G 4 S) 2 The connecting peptide was fused with the N-terminus of the ADH (SEQ ID NO.2) and AmDH (SEQ ID NO.3) genes respectively to obtain CotB1p-ADH and CotB1p-ADH with gene sequences of SEQ ID NO.4 and SEQ ID NO.5 respectively AmDH, and then subcloning the fused gene into the restriction site between NdeI and XhoI of plasmid pColdII (SEQ ID NO.6) to form recombinant plasmids (pColdII-CotB1p-ADH, pColdII-CotB1p-AmDH) , which was verified by sequencing, transformed into Escherichia coli shuffle T7-B competent cells, and obtained genetically engineered strains;
[0084] 2) The genetically engineered strains are cultured in two stages, first grown to OD at 37°C 600nm = 1.0, then add 0.1M IPTG at 16°C to induce recombinant protein expression for 24 hours, collect the expression strain by centrifugation (4000g, 30min) after induction, and store it at -20°C for later use;
[0085] 3) Resuspend the above obtained strain in l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com