Construction and application of amine dehydrogenase mutants with improved thermostability and genetically engineered bacteria
A technology of amine dehydrogenase and thermostability, applied in genetic engineering, application, plant gene improvement, etc., can solve the problems of poor thermostability of amine dehydrogenase, achieve good thermostability, excellent stereoselectivity, good The effect of applying the foreground
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] This embodiment provides an amine dehydrogenase mutant with improved thermostability, wherein the amine dehydrogenase is a wild-type amine dehydrogenase derived from Thermosediminibacter oceani, named ANDD-TDO protein, and encodes the ANDD-TDO protein The nucleic acid sequence is SEQ ID NO.1, and the amino acid sequence is SEQ ID NO.2.
[0049] The amine dehydrogenase mutant with improved thermostability provided in this example comprises: the amino acid sequence shown in SEQ ID NO.2 is substituted, deleted or added with one or more amino acids to form the amino acid sequence shown in SEQ ID NO.2 The amino acid sequence (i.e. ANDD-TDO protein) has the same function as a derivative protein, or the amino acid sequence shown in SEQ ID NO.2 is substituted, deleted or added with one or more amino acids to form the amino acid sequence shown in the SEQ ID NO.2 Derivative proteins with at least 90% homology in amino acid sequence (ie ANDD-TDO protein).
[0050] Specifically, s...
Embodiment 2
[0055] This embodiment provides a method for constructing an amine dehydrogenase mutant with improved thermostability, comprising the following steps:
[0056] 1. Cloning of wild-type amine dehydrogenase ANDD-TDO gene
[0057] The wild-type amine dehydrogenase gene is codon-optimized with Escherichia coli as the host cell, and the optimized ANDD-TDO gene is obtained. Its nucleic acid sequence is SEQ ID NO.1, and the expressed amino acid sequence is SEQ ID NO.2; ID NO.1 is used as the target gene, and the target gene is amplified by using the upstream amplification primer SEQ ID NO.18 and the downstream amplification primer SEQ ID NO.19;
[0058] The nucleic acid sequence of SEQ ID NO.18 is:
[0059] 5'-ACTGCTCATATGGAAAAAATCCGTGTTATCATC-3' (where the underline is the recognition site of restriction endonuclease NdeI);
[0060] The nucleic acid sequence of SEQ ID NO.19 is:
[0061] 5'-TCAGCTCTCGAGTTAAGCGTTGTTAACACCG-3' (the underline is the recognition site of restriction end...
Embodiment 3
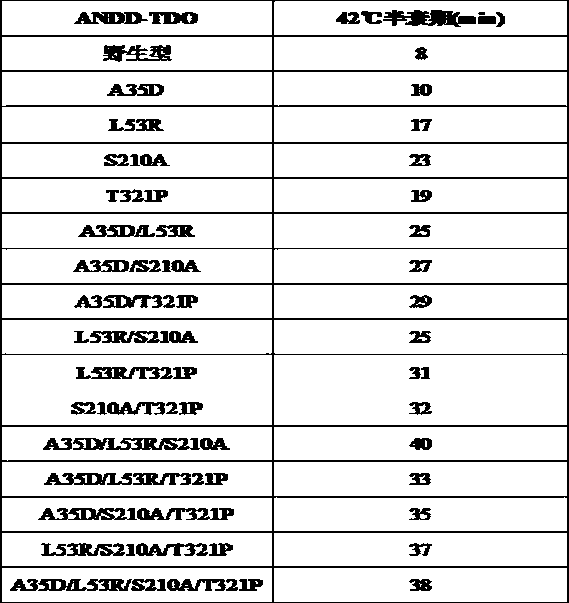
[0127] This example provides a gene encoding the amine dehydrogenase mutant with improved thermostability as described in Example 1:
[0128] (1) The nucleic acid sequence encoding the amine dehydrogenase mutant whose mutation site is A35D is SEQ ID NO.28;
[0129] (2) The nucleic acid sequence encoding the amine dehydrogenase mutant whose mutation site is L53R is SEQ ID NO.29;
[0130] (3) The nucleic acid sequence encoding the amine dehydrogenase mutant whose mutation site is S210A is SEQ ID NO.30;
[0131] (4) The nucleic acid sequence encoding the amine dehydrogenase mutant whose mutation site is T321P is SEQ ID NO.31;
[0132] (5) The nucleic acid sequence encoding the amine dehydrogenase mutant whose mutation site is A35D / L53R is SEQ ID NO.32;
[0133] (6) The nucleic acid sequence encoding the amine dehydrogenase mutant whose mutation site is A35D / S210A is SEQ ID NO.33;
[0134] (7) The nucleic acid sequence encoding the amine dehydrogenase mutant whose mutation site...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com