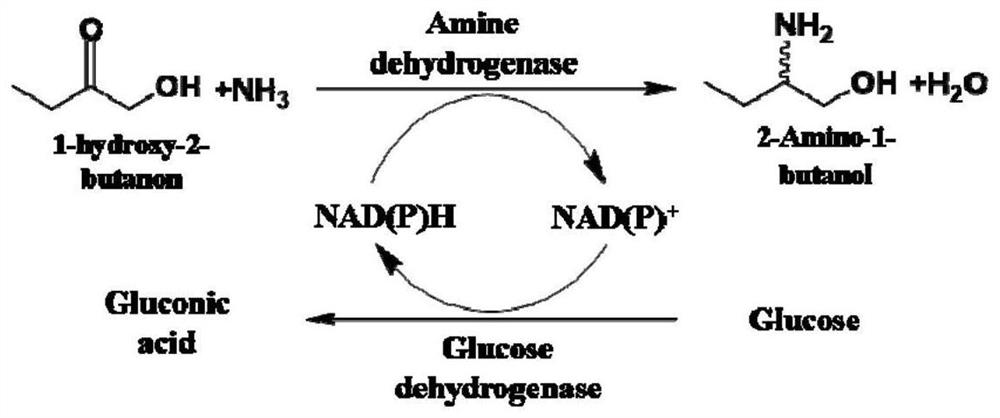
A kind of synthetic method of chiral aminoalcohol compound
A compound, amino alcohol technology, applied in the field of synthesis of chiral amino alcohol compounds, can solve problems such as low safety factor, conversion rate of only 50%, harsh reaction conditions, etc., achieve good industrial application prospects, high optical purity of products, easy operation convenient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
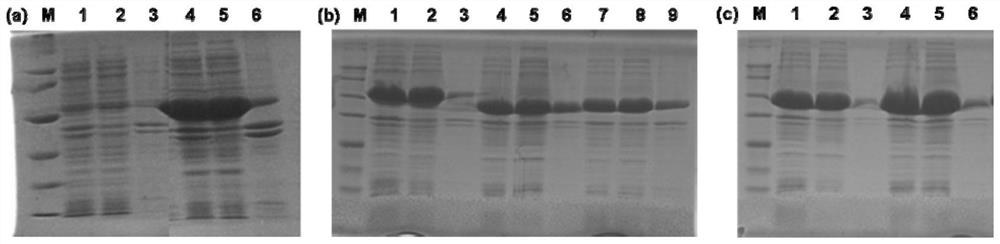
[0082] Example 1. Screening of amine dehydrogenase for biocatalytic preparation of chiral aminoalcohol compounds
[0083] 1. Preparation of recombinant bacteria expressing amine dehydrogenase
[0084] 1. Recombinant plasmid expressing amine dehydrogenase
[0085]The recombinant plasmid expressing amine dehydrogenase is to replace the NdeI and XhoI of the corresponding expression vector (column 3 in Table 2) with the coding gene of each amine dehydrogenase in the amine dehydrogenase library in Table 2 (column 5 of Table 2) A DNA molecule between the sites, resulting in a vector expressing a recombinant protein with a His tag fused to the C-terminus of each amine dehydrogenase.
[0086] The vectors pET22b, pET28a, pET24a, and pET21b all have his tags on them. pET22b, pET21b are ampicillin-resistant, and pET28a, pET24a are kana-resistant.
[0087] Table 2 is the amine dehydrogenase library information table
[0088]
[0089]
[0090]
[0091] 2. Recombinant bacteria e...
Embodiment 2
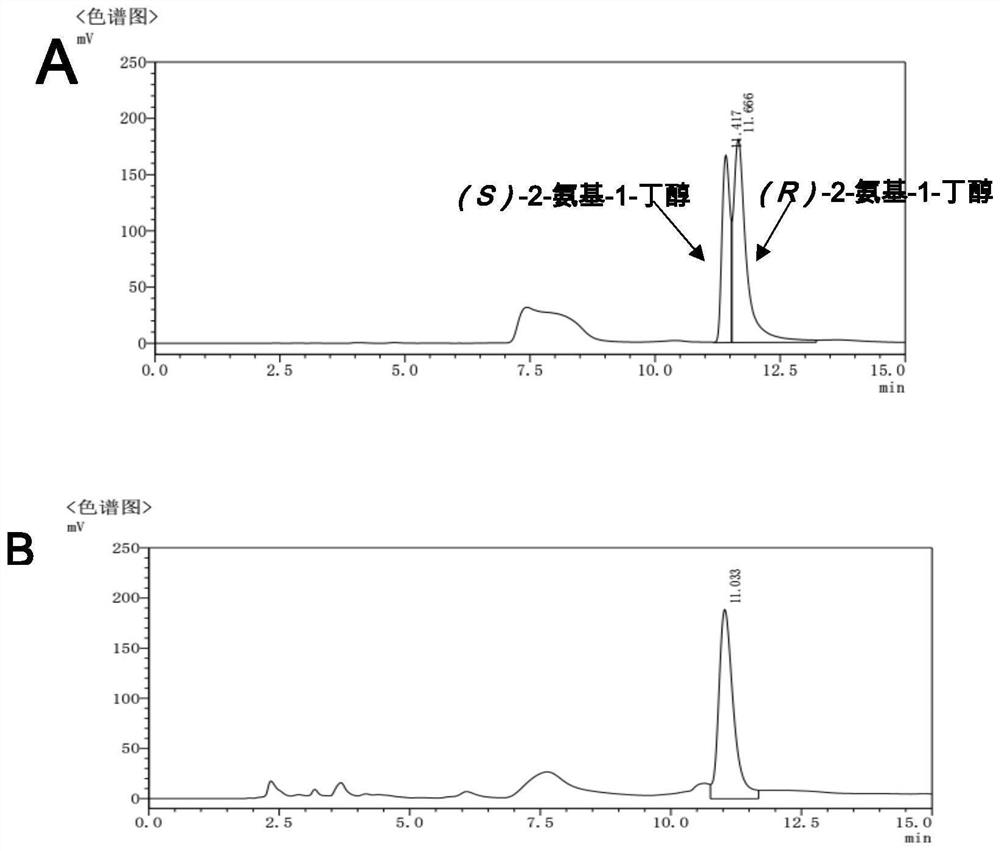
[0127] Example 2, Condition optimization for the preparation of chiral aminoalcohol compounds by amine dehydrogenase biocatalysis
[0128] One, the preparation of pure amine dehydrogenase
[0129] Whole cells expressing recombinant EsAmDH prepared in Example 1, whole cells expressing recombinant BsAmDH1, whole cells expressing recombinant TiAmDH, whole cells expressing recombinant BcAmDH, whole cells expressing recombinant SpAmDH, whole cells expressing recombinant LsAmDH1 The whole cells were ultrasonically disrupted, centrifuged at 12,000rpm, 60min, 4°C to collect the supernatant, filtered through a 0.45μm water filter membrane, and then purified with an ATKA protein purifier. The chromatographic column used was HisTrap HP 5mL prepacked column (GE) , Cat. No. 17524802. Loading and equilibration buffer A (50mM potassium phosphate buffer, 0.5M NaCl, 20mM imidazole, pH 8.0); Elution buffer B (50mM potassium phosphate buffer, 0.5M NaCl, 500mM imidazole, pH 8.0), elution flow ra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com