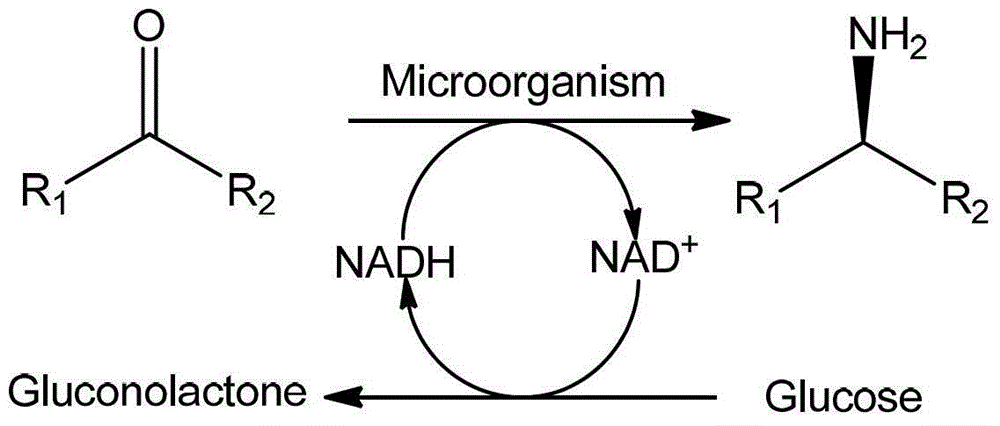
Method for preparing chiral amine through asymmetric reduction under catalysis of marine strain
An asymmetric and chiral amine technology, which is applied in the field of preparation of chiral amines by catalyzing asymmetric reduction of marine strains, can solve the problems of low reaction selectivity, no gene and protein structure, etc., and achieve high yield, simple equipment, and optical purity high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] (1) Cultivation of Pseudomonas kilonsis: Inoculate the strain into 50L216L medium with an inoculation volume of 1%. The composition of 216L medium is 10.0g / L peptone, 5.0g / L yeast powder, 0.5g / L beef extract, 0.5g / L sodium citrate, 0.2g / L NH 4 NO 3 , 1.0g / L NaAc, prepared with sea water. The culture conditions are as follows: initial pH 7.0, volume fraction of liquid volume 10%, culture temperature 30°C, shaker speed 150 rpm, culture time 72 hours.
[0017] Preparation of cells: The fermentation broth obtained at the end of the culture was centrifuged in a refrigerated centrifuge (4°C, 10,000rpm, 15min) to obtain the cells, the supernatant was discarded, the pellet was resuspended with Tris-HCl buffer (pH7.6), and washed thoroughly After centrifugation, the operation was repeated 3 times to obtain cells.
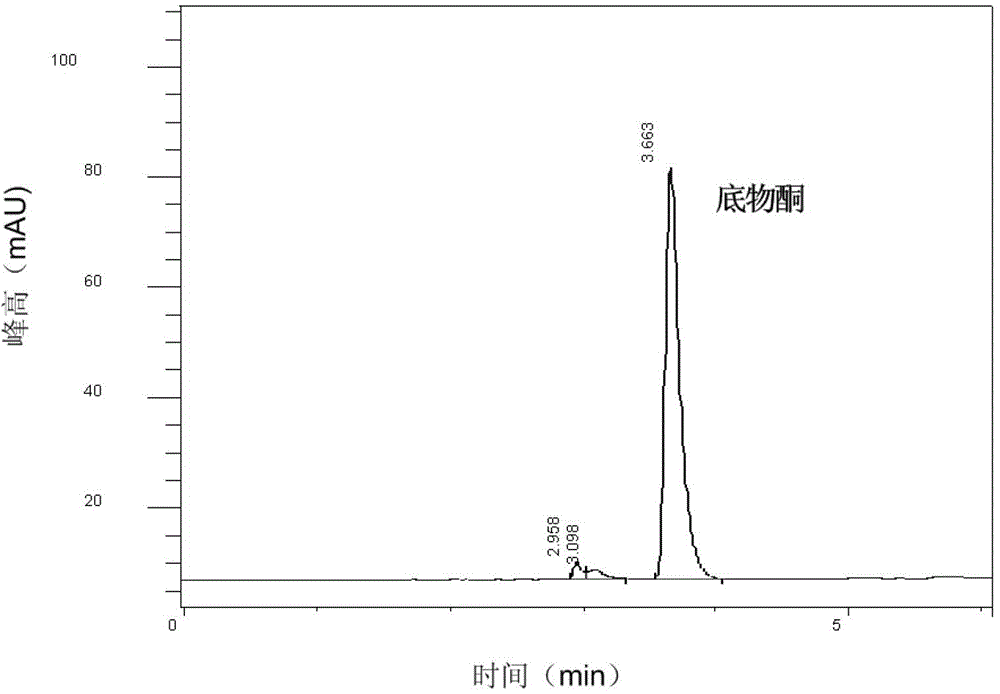
[0018] (2) Whole-cell catalytic reaction: the reaction system is 0.5mol / L 4-methyl-2-pentanone, 1mol / L alanine, 1mol / L glycerol, 0.01mol / L Tris-hydrochloric acid b...
Embodiment 2
[0021] (1) Experimental procedures are as in (1) to (2) of Example 1.
[0022] (2) Whole-cell catalytic reaction: the reaction system is 0.02mol / L 2-butanone, 0.2mol / L NH 4 Cl, 0.2mol / L glucose, 0.01mol / L sodium acetate buffer, 1.5g / L cells, reaction system pH 13, reaction volume 500mL, at 50°C, 200rpm shaking reaction, reaction time 30h.
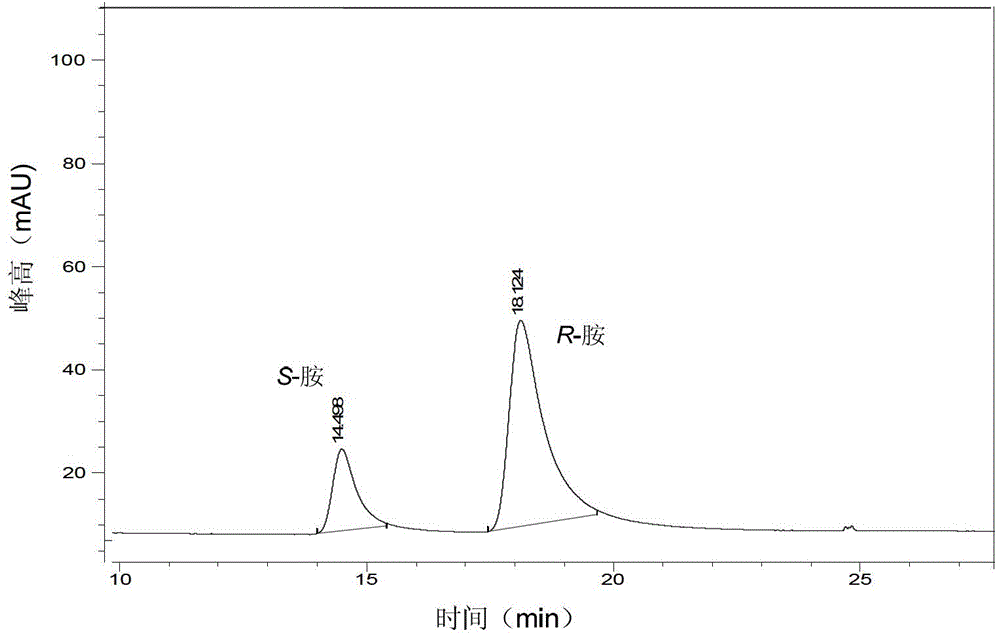
[0023] (3) Separation and detection: Use 6mol / L NaOH to adjust the pH to 14, then extract with 250mL of methyl tert-butyl ether, the separated methyl tert-butyl ether layer is dehydrated with anhydrous sodium sulfate, vacuum at low temperature Dry, dissolve the residual solid with a mixed solution of n-hexane and isopropanol (volume ratio is 9:1), detect with high performance liquid chromatography, calculate the yield of product amine R-2-butylamine to be 72.1%, e.e. value was 92.7%.
Embodiment 3
[0025] (1) Experimental procedures are as in (1) to (2) of Example 1.
[0026] (2) Whole-cell catalytic reaction: the reaction system is 0.01mol / L acetophenone, 0.02mol / L ammonia water, 0.01mol / L isopropanol, 0.2mol / L Tris-hydrochloric acid buffer, 0.025g / L cells , the reaction volume is 20L, the pH of the reaction system is 7, the reaction volume is 5L, the reaction is shaken at 10°C at 300rpm, and the reaction time is 180h.
[0027] (3) Separation and detection: Use 6mol / L NaOH to adjust the pH to 14, and then extract with 10L methyl tert-butyl ether. The separated methyl tert-butyl ether layer is dehydrated with anhydrous sodium sulfate, and vacuumed at low temperature After drying, the residual solid was dissolved with a mixture of n-hexane and isopropanol (9:1 by volume), and detected by high performance liquid chromatography. The yield of the product S-phenylethylamine was 80.7%, and the e.e. value was 90.2%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com