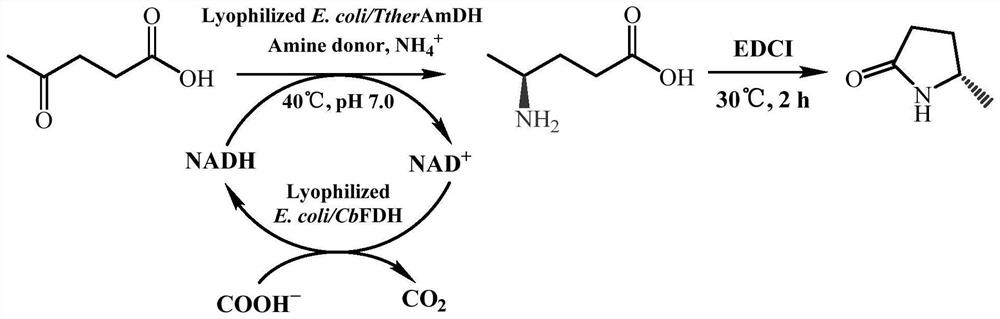
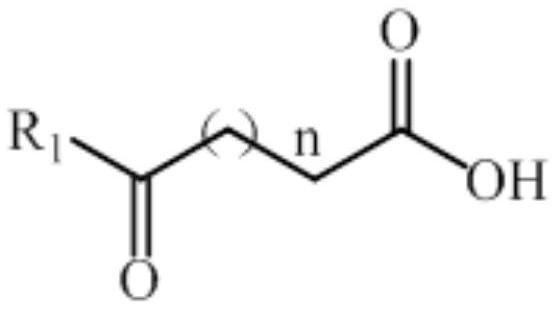
Amine dehydrogenase mutant and application thereof in preparation of (S)-5-methyl-2-pyrrolidone
An amine dehydrogenase, mutant technology, applied in the application, genetic engineering, oxidoreductase and other directions, can solve the problems of mild reaction environment, moderate stereoselectivity, low enzyme activity, etc., achieve good industrial application prospects, reduce Coenzyme dosage and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Screening of TtherAmDH mutants with increased activity by random mutagenesis
[0077] TtherAmDH containing the amino acid sequence shown in SEQ ID No. 2 was randomly mutated using error-prone PCR technology.
[0078] The primers used were:
[0079] Upstream primer, as shown in SEQ ID No.3:
[0080] CCG GAATTC ATGGAAAATATAAAAGTCGTAGTTTGGGG
[0081] Downstream primer, as shown in SEQ ID No.4:
[0082] CCC AAGCTT TTAACGGCGACGAATCATAT
[0083] The sequence underlined in the upstream primer is the restriction site of EcoR I, and the sequence underlined in the downstream primer is the restriction site of Hind III.
[0084] Using pET28a-TtherAmDH as a template, error-prone PCR was performed with rTaq DNA polymerase to construct a random mutation library. PCR system (50 μL): rTaq DNA polymerase 0.5 μl, 10× PCR buffer (Mg 2+ Plus) 5.0 μl, dNTP Mixture (2.0 mM each) 4.0 μl, MnCl with a final concentration of 250 μmol / L 2 , pET28a-TtherAmDH plasmid 100ng, upstream and d...
Embodiment 2
[0087] Example 2 Semi-rational construction of TtherAmDH mutant
[0088] In Example 1, using error-prone PCR technology and high-throughput screening method, a mutation site (F24K) with significantly improved catalytic activity and soluble expression was successfully screened. Homologous modeling was performed on TtherAmDH, and then the modeled mutant model was used for molecular docking with levulinic acid. According to the catalytic mechanism and binding energy of amine dehydrogenase, an appropriate docking posture model was selected. The activity of the enzyme was further enhanced by site-directed saturation mutation and combinatorial mutation of amino acids near the substrate pocket. In the three-dimensional structure of TtherAmDH of the amino acid sequence shown in SEQ ID No.2, the amino acid residues around the levulinic acid binding site of the substrate are mainly selected from the amino acid sites on the surrounding loops. Residues were mutated by saturation.
[008...
Embodiment 3
[0109] Example 3 Recombinant E. coli BL21(DE3) / pET28a-TtherAmDH V9 expression and enzymatic preparation of
[0110] The recombinant E. coli BL21(DE3) / pET28a-TtherAmDH of the mutant V9 obtained in Example 2 was V9 Inoculated into LB medium containing 50μg / ml kanamycin, shaken at 37°C for 12h, and then inserted into 100ml LB medium (containing 50μg / ml card) at a 1% (v / v) inoculum amount. Namycin) in a 500ml Erlenmeyer flask, placed at 37°C and shaken at 220rpm for culture, when the OD600 of the culture medium reached 0.6, IPTG with a final concentration of 0.2mmol / L was added as an inducer, and induced at 16°C for 24h. The culture medium was centrifuged at 8000 × g for 10 min, the cells were collected, and washed twice with normal saline to obtain resting cells. The cells obtained in 100 ml of culture medium were suspended in 15 ml of potassium phosphate buffer (100 mM, pH 7.0), and the following ultrasonic disruption was performed in an ice-water bath: 350 W power, work for 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com