A method for preparing chiral amines catalyzed by a marine strain and its amine dehydrogenase
A technology for catalytic preparation of chiral amines, applied in biochemical equipment and methods, microorganism-based methods, microorganisms, etc., can solve the problems of low reaction selectivity, no gene and protein structure, etc., and achieve high yield and simple equipment. , the effect of convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] 1) Cultivation of strains: Pseudomonas balearica (Pseudomonas balearica) cultivation: Pseudomonas balearica (P.balearica) with 1% (ml / ml) inoculum size, bacterial classification is inserted into 50mL 216L culture medium. The composition of 216L medium is 10.0g / L peptone, 5.0g / L yeast powder, 0.5g / L beef extract, 0.5g / L sodium citrate, 0.2g / L NH 4 NO 3 , 1.0g / L sodium acetate, configured with sea water. The culture conditions are as follows: the initial pH is 7.0, the volume fraction of the filling volume is 10%, the culture temperature is 30° C., the shaking table rotates at 150 rpm, and the culture time is 72 hours.
[0048] 2) Preparation of whole cells: centrifuge the fermented liquid obtained in step 1) in a refrigerated centrifuge (4°C, 10000rpm, 15min) to obtain cells, discard the supernatant, and use Tris-HCl buffer (pH 7.6 for precipitation) ) resuspended, fully washed and then centrifuged, repeating the above precipitation resuspension-washing-centrifugation...
Embodiment 2
[0053] 1)-2) The experimental steps are as in the steps 1)-2) of Example 1, and in step 2), the cells are prepared with 0.2 mol / L potassium phosphate buffer solution of pH 10 to prepare 2500 mg / L cell fluid;
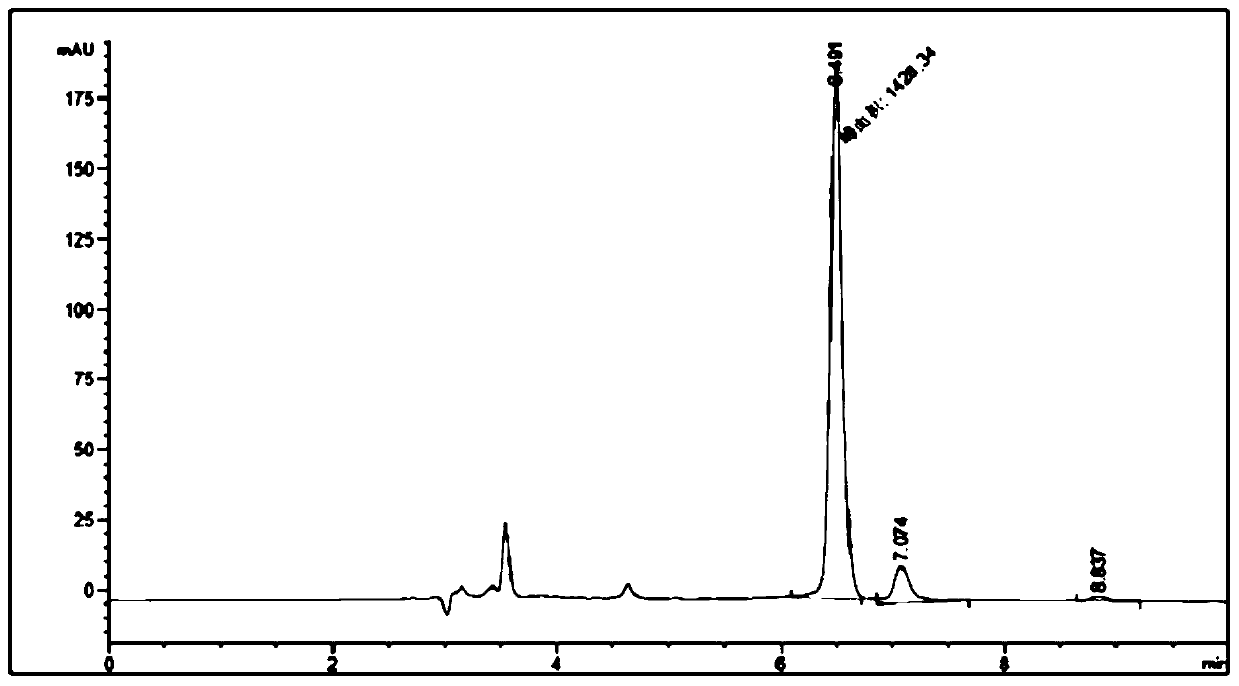
[0054] 3) Whole-cell catalysis: Based on the 2500mg / L cell fluid obtained in step 2), prepare a whole-cell catalytic system: 0.01mol / L 5-decanone, 0.01mol / L 2-aminopropane, 0.02mol / L sec-butyl Alcohol, 0.2mol / L potassium phosphate buffer, pH 10, 2500mg / L cells, reaction volume 500mL, reaction system pH 10, reaction temperature 50°C, 300rpm shaking reaction for 70 hours, using the whole cell to catalyze asymmetric reduction Amination affords R-5-decylamine.
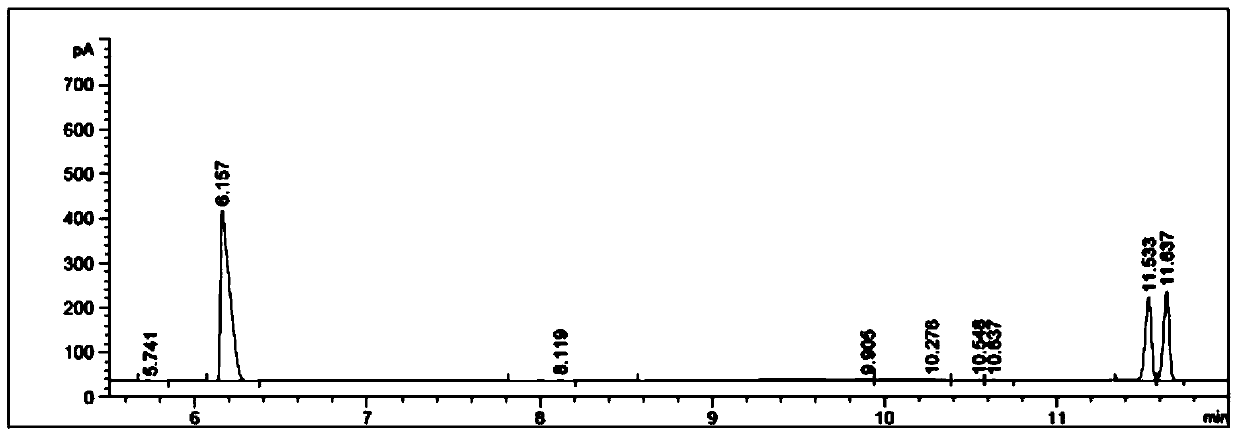
[0055] 4) Separation and detection: Separation was carried out according to step 4) in Example 1, and detected by high performance liquid chromatography, the yield of product amine was 60.1%, and the e.e. value was 95.7%.
Embodiment 3
[0057] 1)-2) The experimental steps are as in the steps 1)-2) of Example 1, and in step 2), the cells are prepared with 0.2 mol / L Tris-hydrochloride buffer solution of pH 7.5 to prepare 25 mg / L cell fluid;
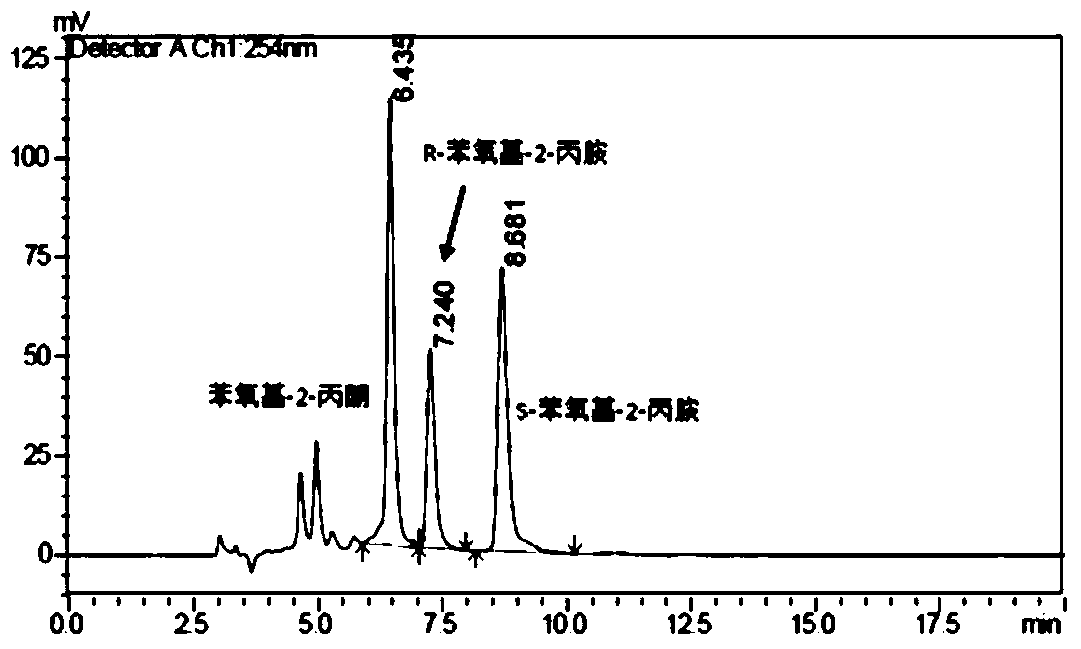
[0058] 3) Whole-cell catalysis: Based on the 25mg / L cell fluid obtained in step 2), prepare a whole-cell catalytic system: 0.01mol / L acetophenone, 0.02mol / L ammonia water, 0.01mol / L isopropanol, 0.2mol / L Tris-HCl buffer solution, 25mg / L cells, reaction system pH 7.5, reaction volume 5L, reaction temperature 10°C, 300rpm shaking reaction, reaction time 180 hours, using the whole cell to catalyze asymmetric reductive amination to obtain The product R-phenethylamine.
[0059] 4) Separation and detection: Separation was carried out according to step 4) in Example 1, and detected by high performance liquid chromatography, the yield of product amine was 91.7%, and the e.e. value was 93.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com