Amine Dehydrogenase Mutants and Their Applications in the Synthesis of Chiral Amino Alcohols
A technology of alcohol compounds and chiral amines, which can be used in applications, methods based on microorganisms, and the use of vectors to introduce foreign genetic materials, etc., can solve problems such as low conversion rate, low safety factor, and large pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
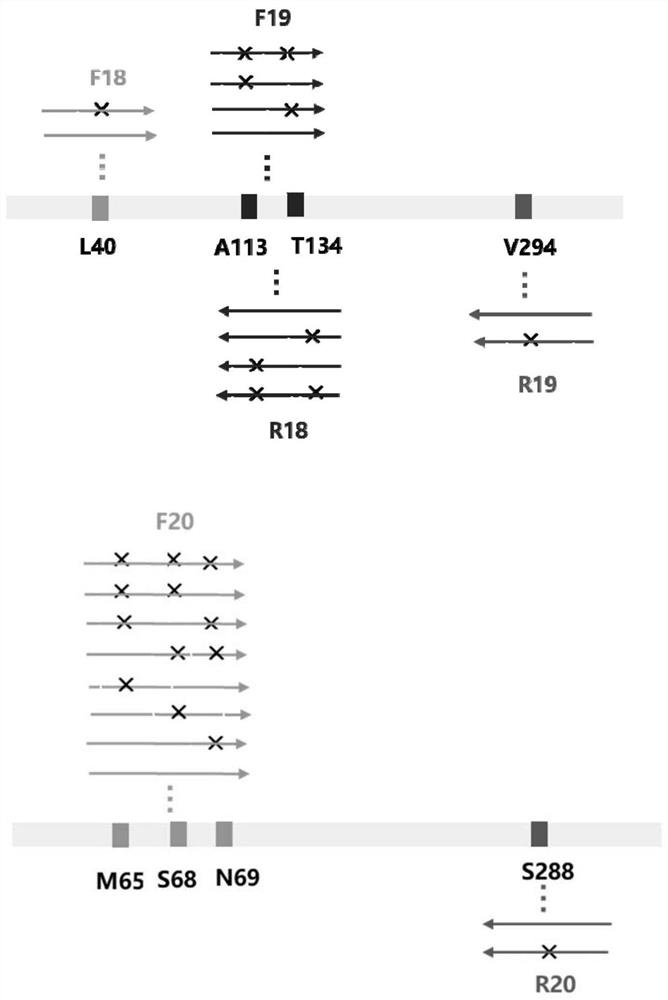
Image
Examples
Embodiment 1
[0202] Embodiment 1, the preparation of the engineering bacterium of amine dehydrogenase SpAmDH gene or its mutant
[0203] 1. Preparation of amine dehydrogenase SpAmDH genetic engineering strain
[0204] Codon-optimized the coding gene of amine dehydrogenase (SpAmDH) derived from Sporosarcina psychrophila using Escherichia coli as a host cell to obtain the SpAmDH gene shown in SEQ ID No.1 in the sequence listing. The amino acid sequence of SpAmDH is shown as SEQ ID No.2 in the sequence listing.
[0205] The SpAmDH gene shown in SEQ ID No.1 in the whole gene synthesis sequence list, the small DNA fragment between NdeI and XhoI recognition sequences in the pET24a (+) vector (Novagen) is replaced by the SpAmDH gene shown in SEQ ID No.1, A recombinant vector was obtained, which was designated as pET24a-SpAmDH, pET24a-SpAmDH can express SpAmDH shown in SEQ ID No.2 in the sequence table, and the expression of the SpAmDH gene is driven by the T7 promoter.
[0206] The recombinant ...
Embodiment 2
[0239] Example 2, Expression of amine dehydrogenase SpAmDH or its mutants and preparation of whole cells, crude enzyme powder and pure enzyme solution
[0240] The recombinant bacteria BL21(DE3) / pET24a-SpAmDH, BL21(DE3) / pET24a and each amine dehydrogenase SpAmDH gene mutant engineering strain prepared in Example 1 were induced to express, and the whole cells and crude enzyme powder of each strain were obtained With pure enzyme solution, the operation steps of each strain are as follows:
[0241] Pick the strain into 5mL LB liquid medium containing 50μg / mL kanamycin, shake overnight at 37°C and 220rpm for 12h, and then inoculate the TB containing 50μg / mL kanamycin according to the volume percentage content of 1% inoculum Cultured in liquid medium at 37°C to OD 600 When the concentration is 0.7, add IPTG with a final concentration of 0.1mmol / L, induce expression at 20°C and 220rpm for 12h, centrifuge at 4°C and 4,000rpm for 10min, collect the precipitated cells (i.e. whole cell...
Embodiment 3
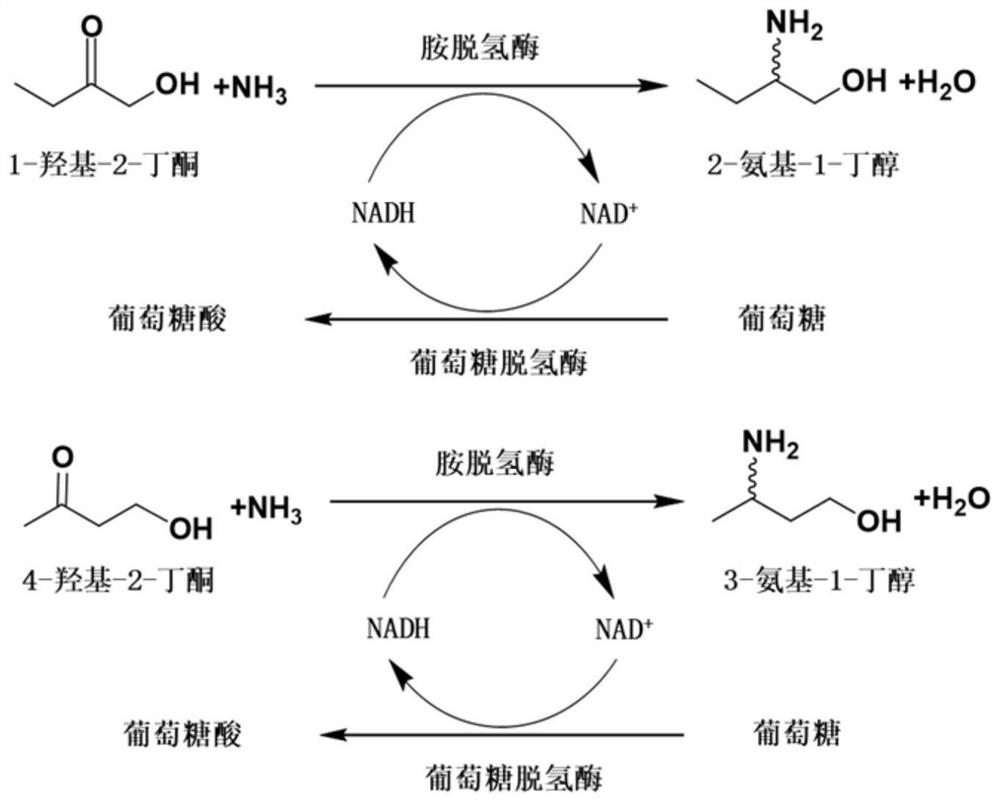
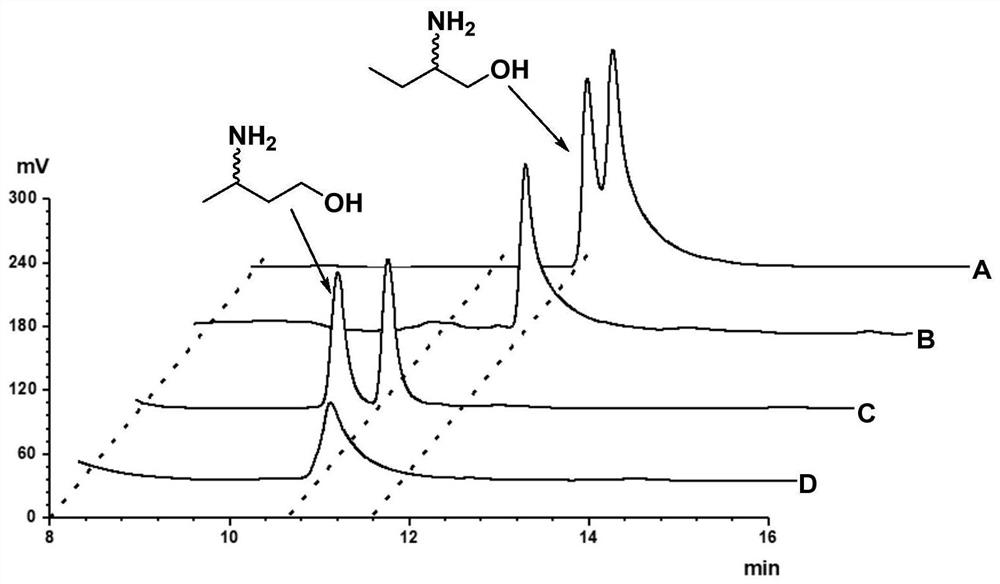
[0242] Example 3. Amine dehydrogenase SpAmDH or its mutants catalyze 1-hydroxy-2-butanone to generate (S)-2-amino-1-butanol
[0243] Use the crude enzyme powder or whole cells of the amine dehydrogenase SpAmDH prepared in Example 2 or its mutants to catalyze 1-hydroxy-2-butanone to generate (S)-2-amino-1-butanol, the schematic diagram is as follows figure 2 shown, and used BL21(DE3) / pET24a as a control.
[0244] The reaction system of the catalyzed reaction of SpAmDH or its mutants is obtained by adding the following amount of substances to 1 mol / L ammonium chloride / ammonia aqueous buffer (ammonium chloride and ammonia aqueous equimolar ratio are mixed, pH 8.5): Substrate 1 -Hydroxy-2-butanone 20mmol / L or 40mmol / L, amine dehydrogenase SpAmDH or its mutant crude enzyme powder 20g / L or whole cell 100g / L, NAD + (in the form of oxidized coenzyme I aqueous solution) 1mmol / L, GDH crude enzyme powder 2g / L, glucose 100mmol / L, lysozyme 1g / L (Beijing Solaibao Technology Co., Ltd., CAS...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com