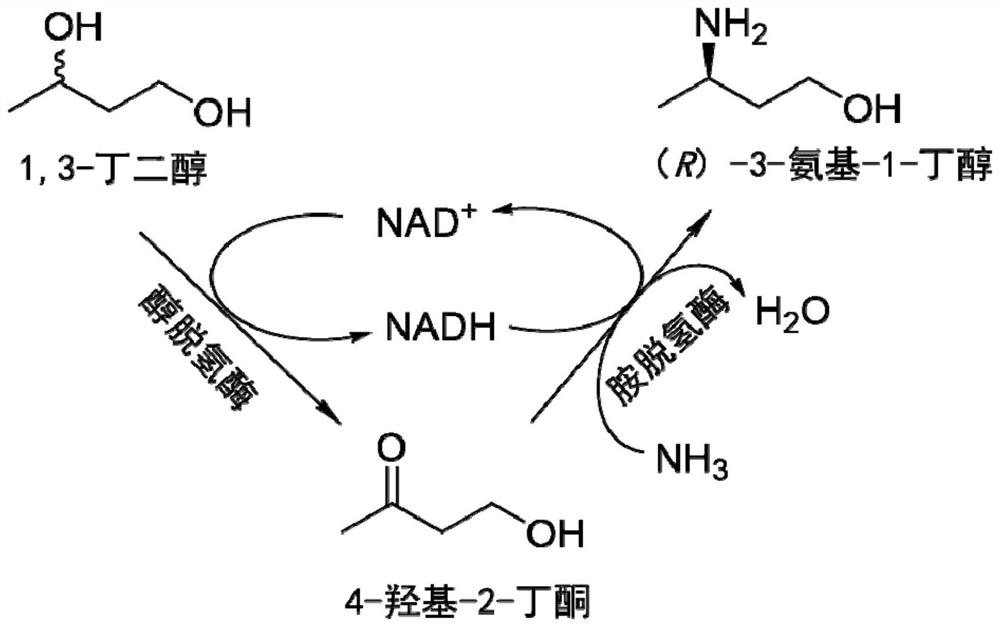
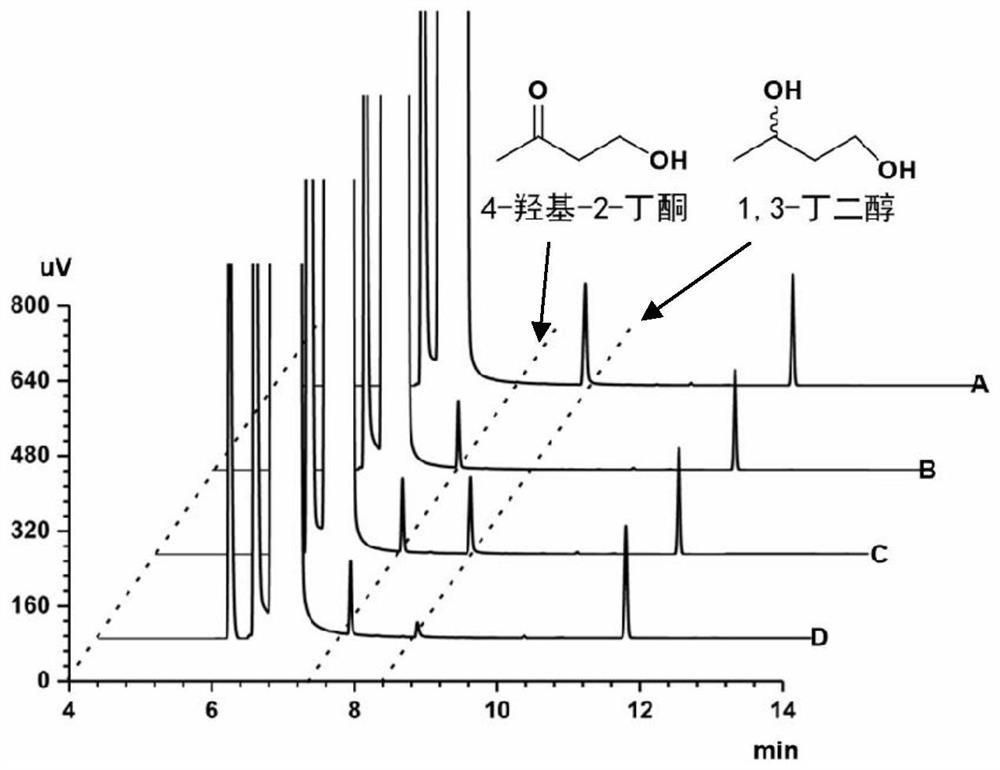
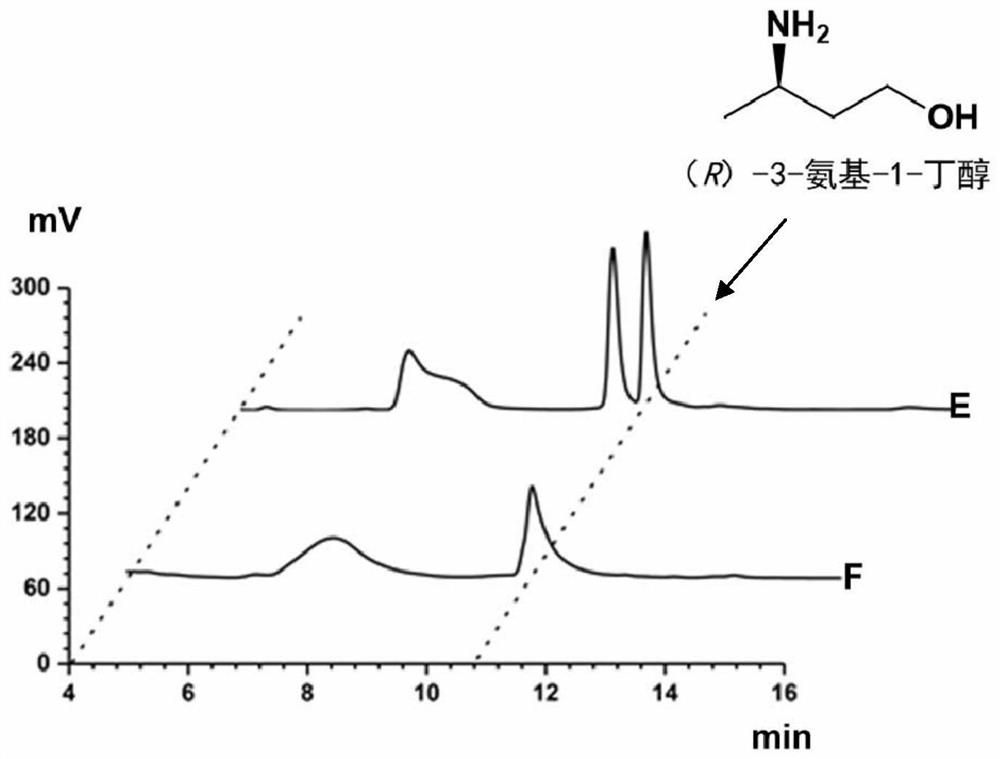
Method for synthesizing (R)-3-amino-1-butanol through double-enzyme cascade catalysis
A technology of amino and butanol, applied in the biological field, can solve problems such as unsuitable for large-scale industrial production, difficult separation and purification, and low safety factor, and achieve good industrial application prospects, high optical purity of products, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0115]Embodiment 1, the preparation of engineering bacteria expressing alcohol dehydrogenase and engineering bacteria expressing amine dehydrogenase
[0116] The genes encoding alcohol dehydrogenase and amine dehydrogenase were respectively sent to Jinkairui Biotechnology Co., Ltd. for synthesis (codon optimization was performed with Escherichia coli as the host as required), and the synthesized genes were connected to various expression vectors to construct become. The expression vectors are various conventional vectors in the art. The vector of the present invention is specifically pET28a, and the small DNA fragment between NcoI and XhoI of the pET28a restriction recognition site is replaced with the coding gene of the related enzyme after the whole gene synthesis to obtain the recombinant expression vector.
[0117] The above recombinant expression vectors are transformed into suitable microbial hosts. The host microorganisms are various conventional host microorganisms i...
Embodiment 2
[0120] Embodiment 2, the preparation of the engineering bacterium that alcohol dehydrogenase and amine dehydrogenase co-express
[0121] The coding genes of alcohol dehydrogenase (or alcohol dehydrogenase mutant) and amine dehydrogenase (or amine dehydrogenase mutant) were respectively subjected to whole gene synthesis (codon optimization was performed with Escherichia coli as the host as required), It is constructed by linking the synthetic gene to various expression vectors. The expression vectors are various conventional vectors in the art. The vector of the present invention is specifically pET28a. Firstly, the small DNA fragment between NcoI and XhoI of pET28a's enzyme-cleaved recognition sites was replaced with the coding gene of alcohol dehydrogenase after the whole gene synthesis to obtain the intermediate vector; Co., Ltd., Cat. No. C112-02) after the whole gene synthesis, the coding gene of amine dehydrogenase was connected to the coding gene of alcohol dehydrogena...
Embodiment 3
[0123] Example 3. Expression or co-expression of alcohol dehydrogenase and amine dehydrogenase and preparation of whole cells and crude enzymes
[0124] 1. Transform the recombinant expression vector constructed in Example 1 or the recombinant expression plasmid constructed in Example 2 into Escherichia coli BL21 (DE3) competent cells to obtain recombinant cells.
[0125] 2. Pick the transformant into 5mL LB liquid medium containing 50μg / mL kanamycin, shake overnight at 37°C and 220rpm for 12h, and then inoculate into 50μg / mL kanamycin containing 50μg / mL kanamycin cultured at 37°C to OD in plain TB liquid medium 600nm When the concentration is 0.7, add IPTG with a final concentration of 0.1mmol / L, induce expression at 20°C and 220rpm for 12h, centrifuge at 4°C and 4000rpm for 10min, collect the precipitated cells (i.e. whole cells), and use the collected cells with phosphate Buffer (50mmol / L, pH 7.4) was resuspended to obtain a bacterial suspension; then the bacterial cells w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com