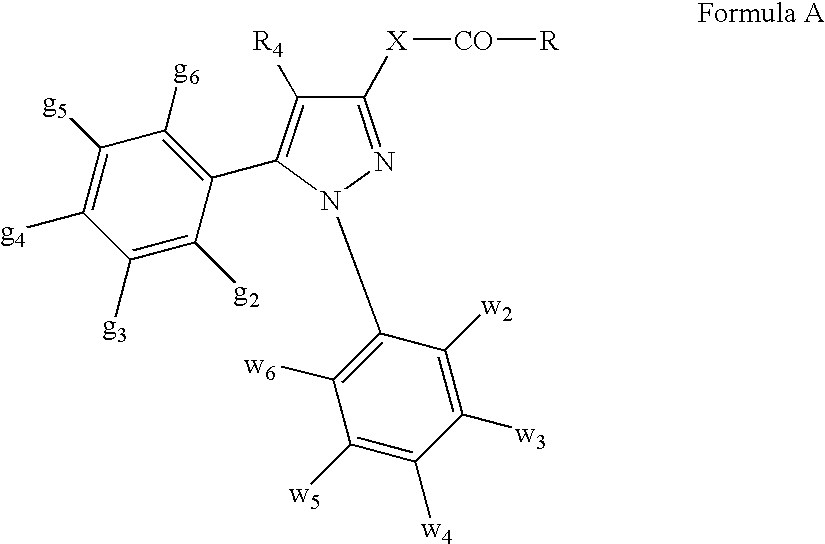
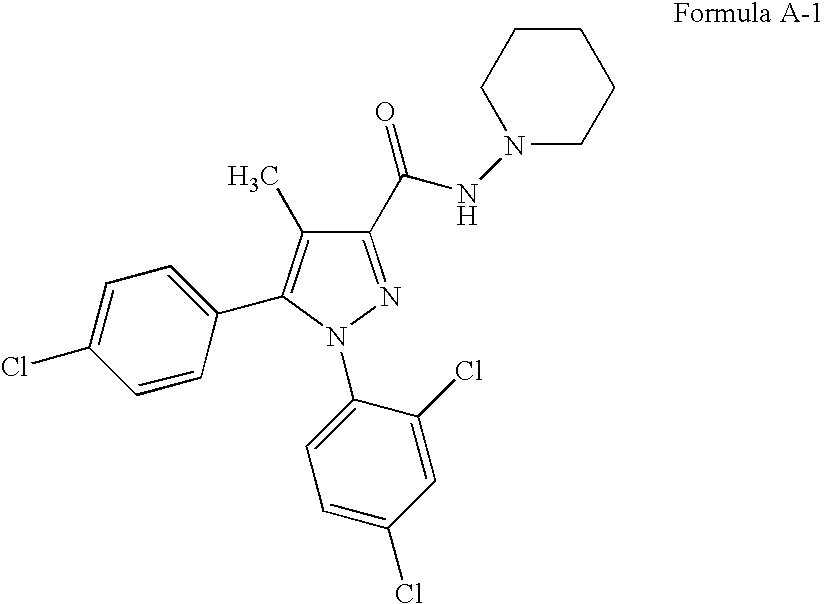
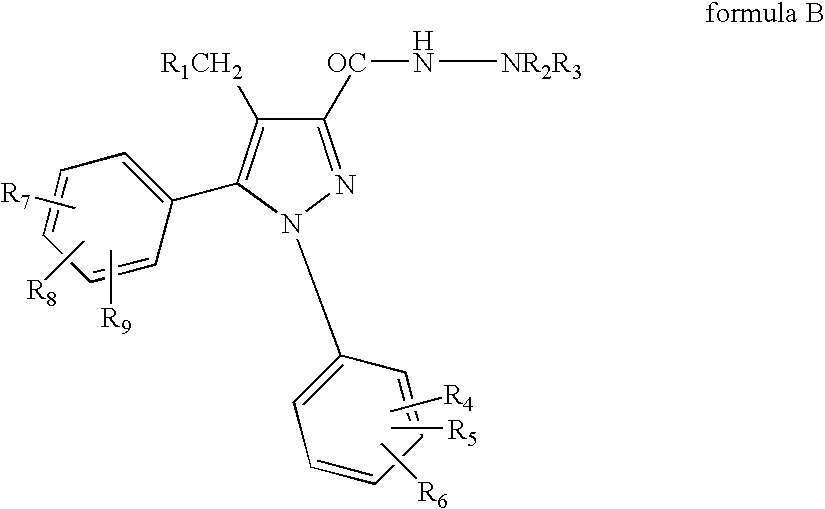
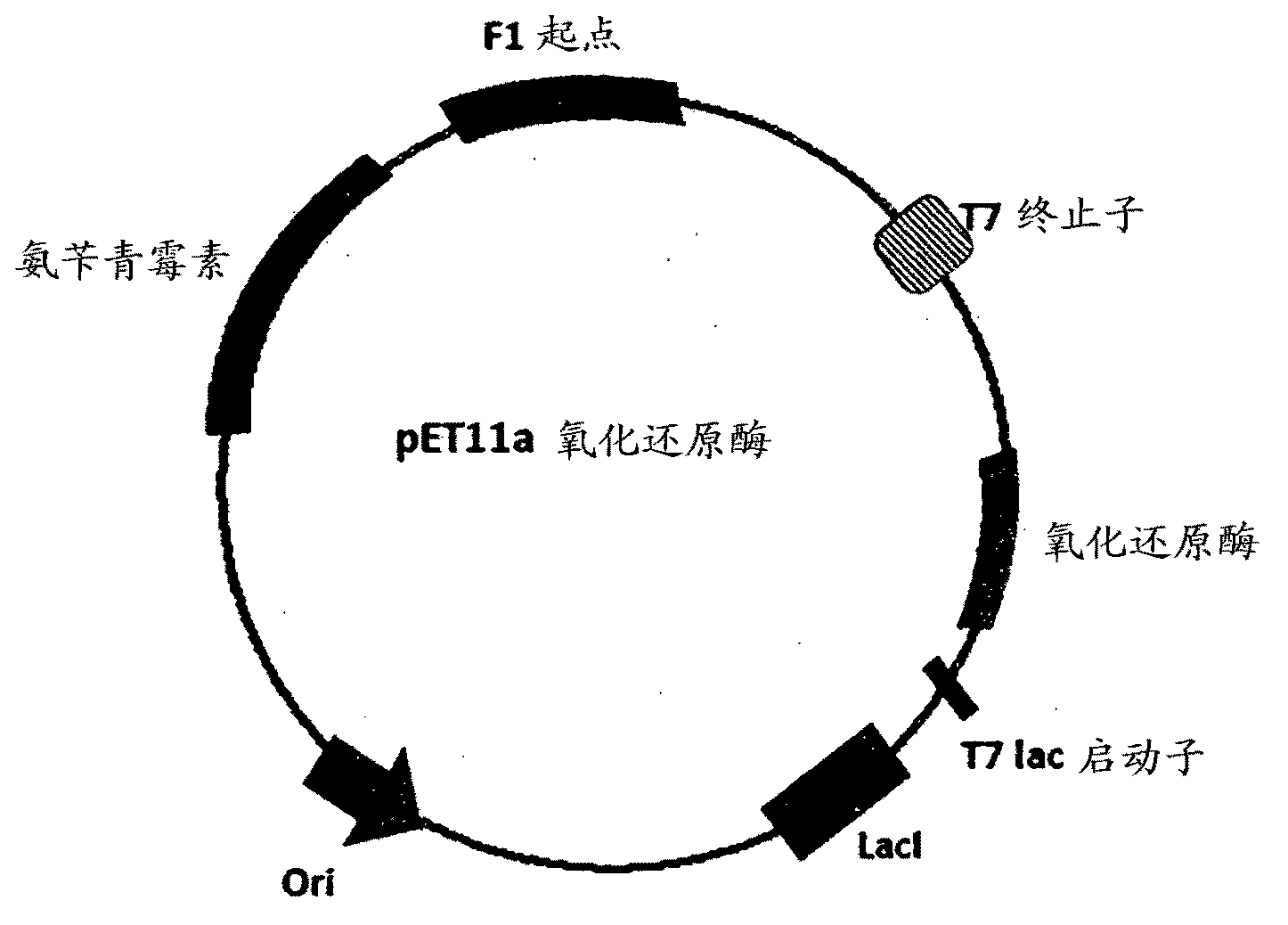
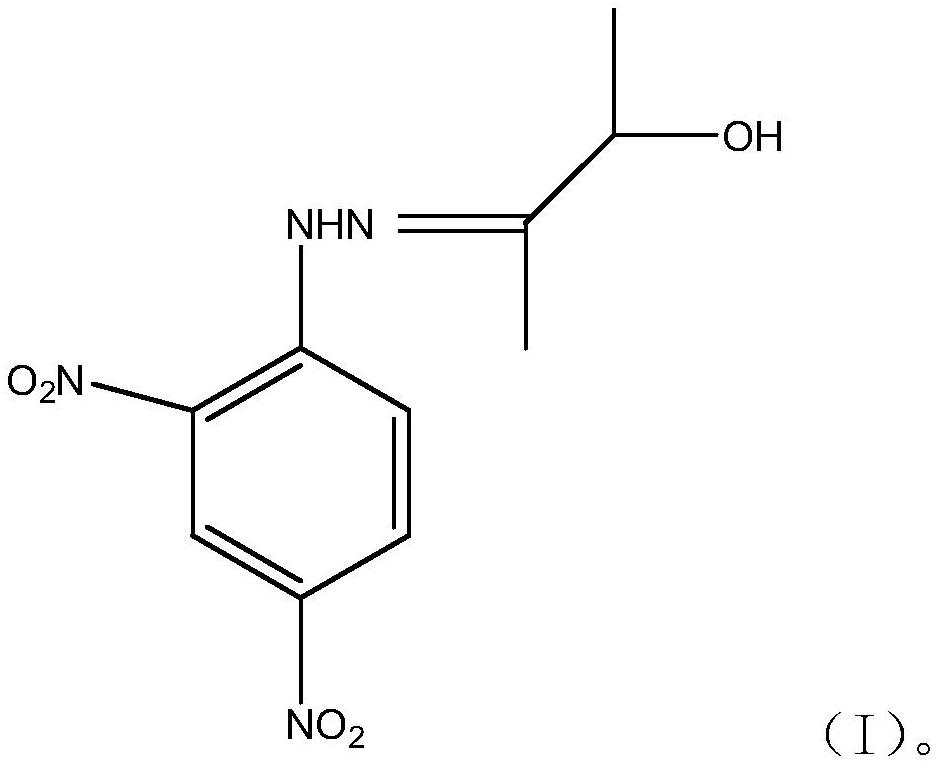
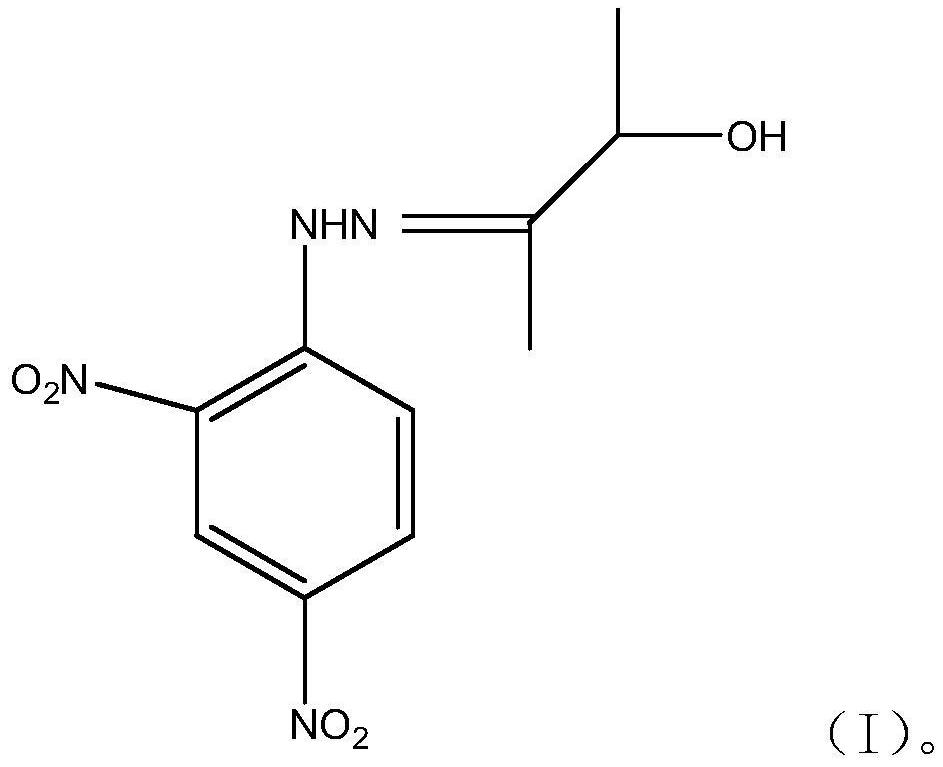
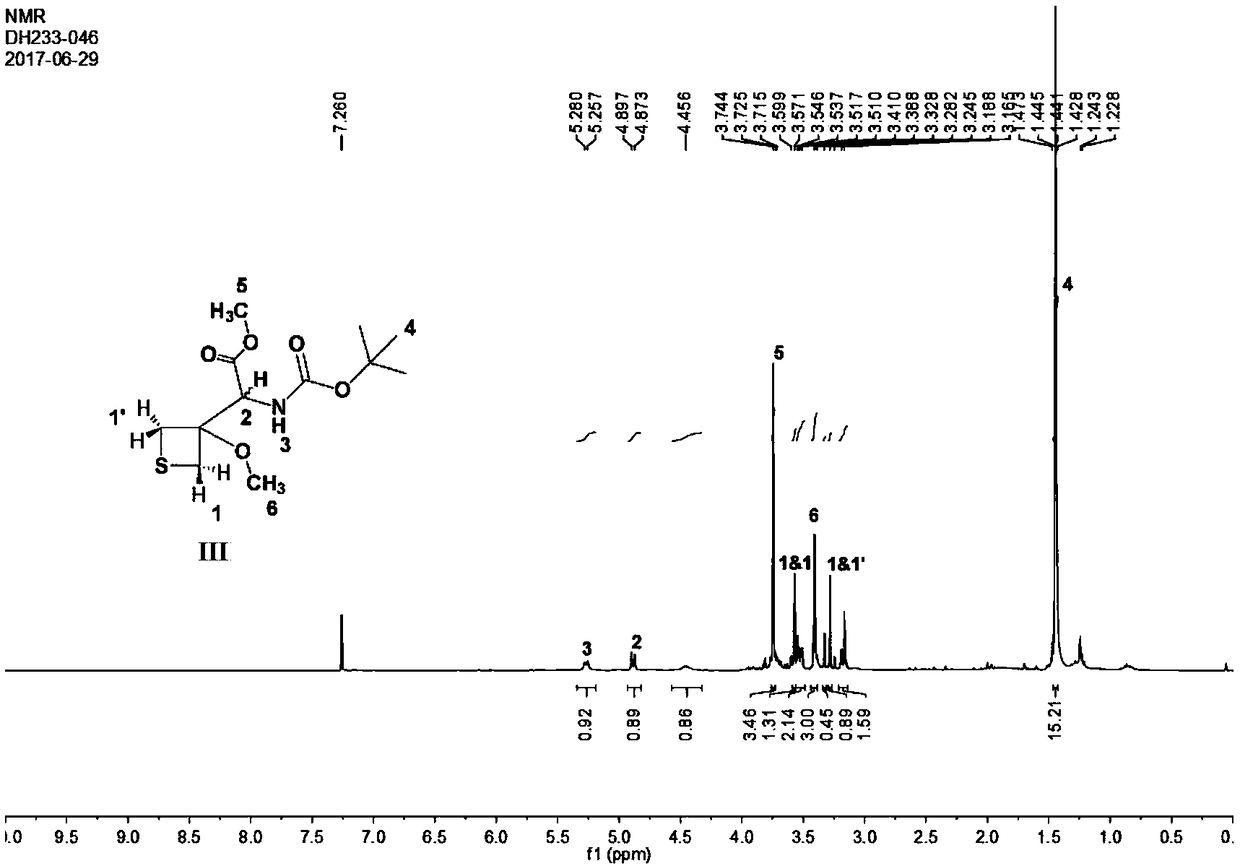
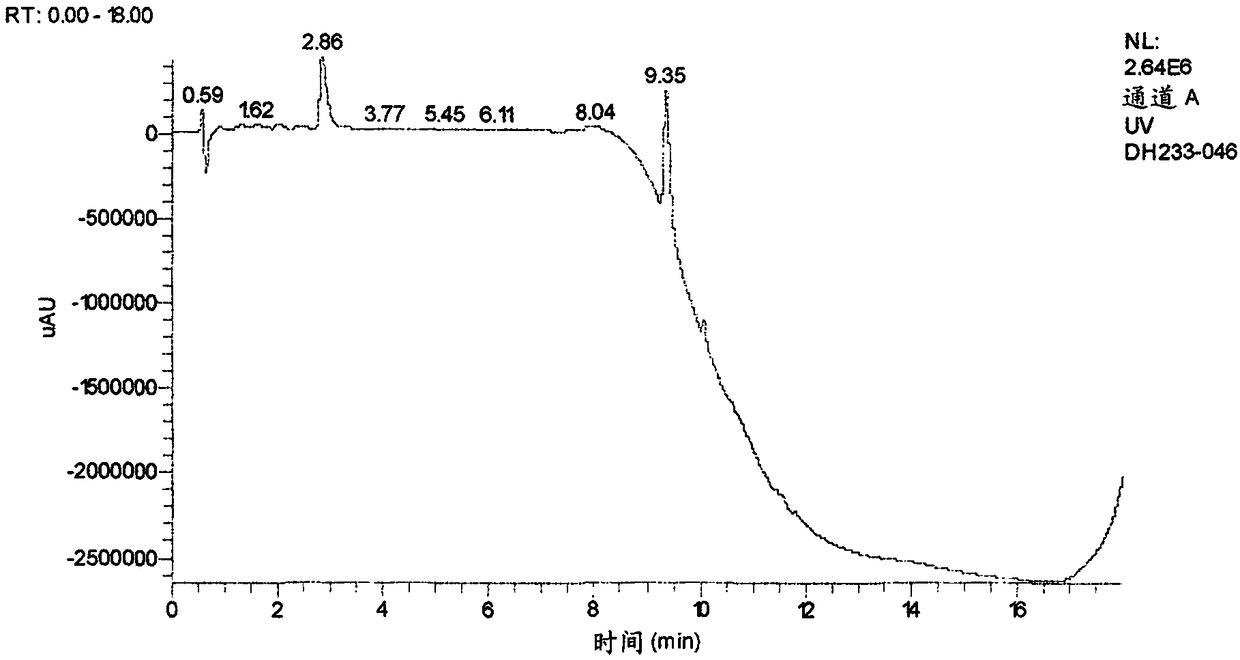
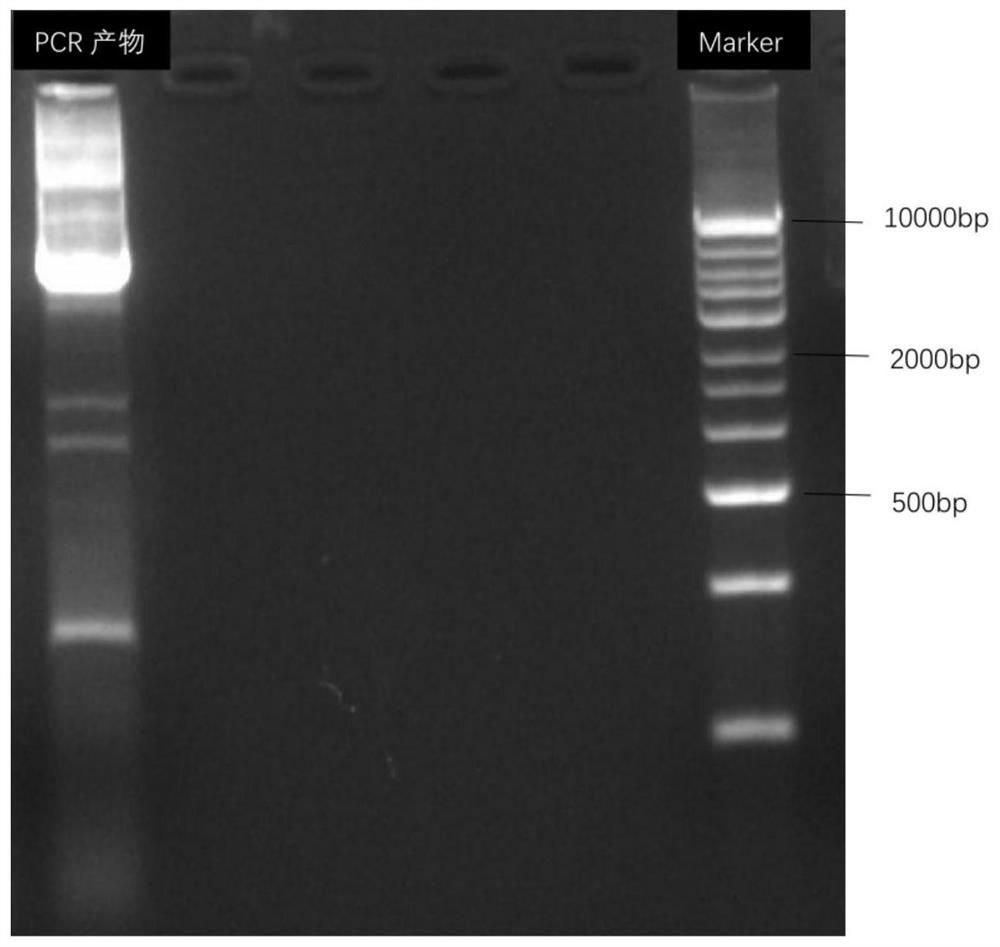
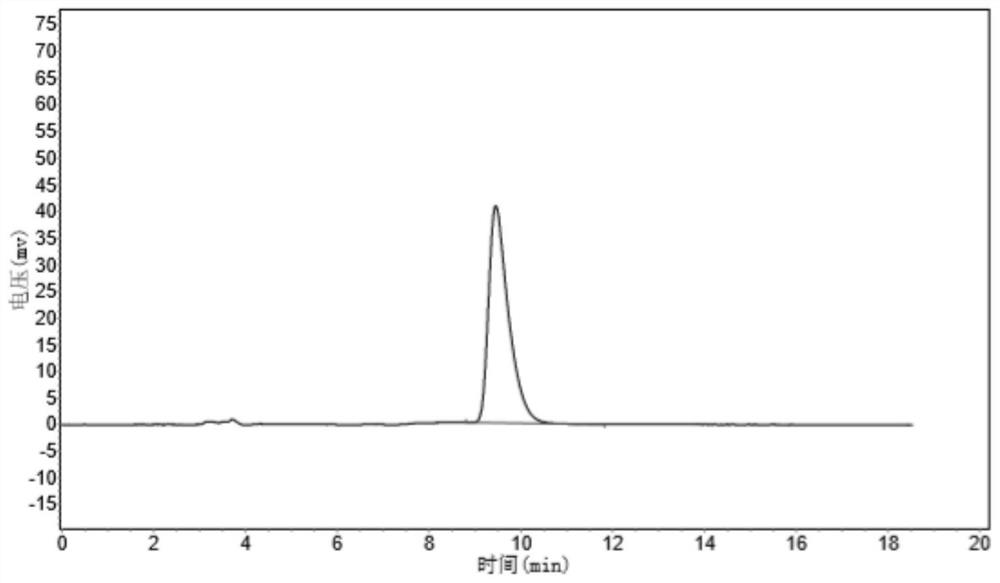
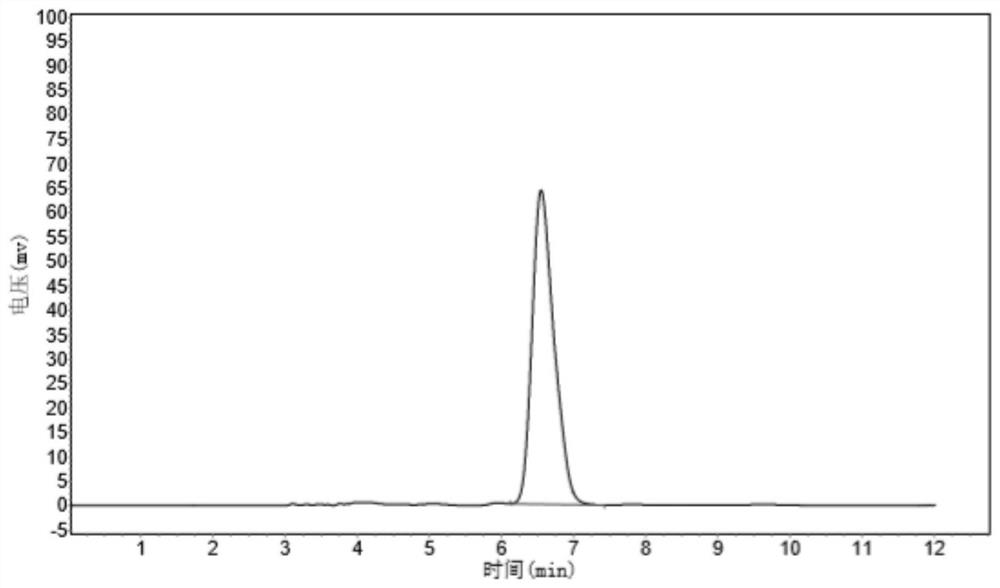
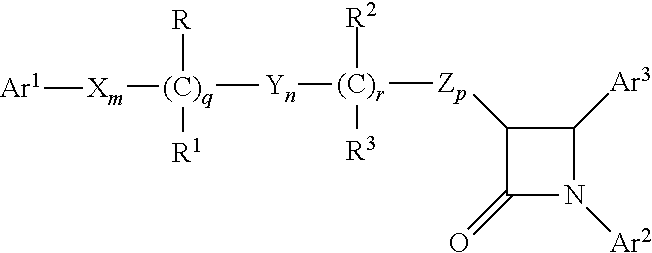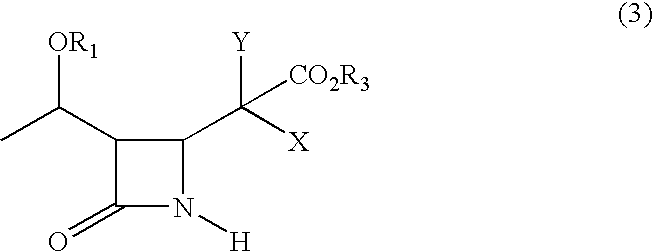Patents
Literature
76 results about "Butylone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Butylone, also known as β-keto-N-methylbenzodioxolylbutanamine (βk-MBDB), is an entactogen, psychedelic, and stimulant psychoactive drug of the phenethylamine chemical class. It is the β-keto (substituted cathinone) analogue of MBDB and the substituted methylenedioxyphenethylamine analogue of buphedrone.
Combinations of substituted azetidinones and CB1 antagonists
The present invention provides compositions, therapeutic combinations and methods including: (a) at least one selective CB1 antagonist; and (b) at least one substituted azetidinone or substituted β-lactam sterol absorption inhibitor which can be useful for treating vascular conditions, diabetes, obesity, metabolic syndrome and lowering plasma levels of sterols or 5α-stanols.
Owner:SCHERING CORP
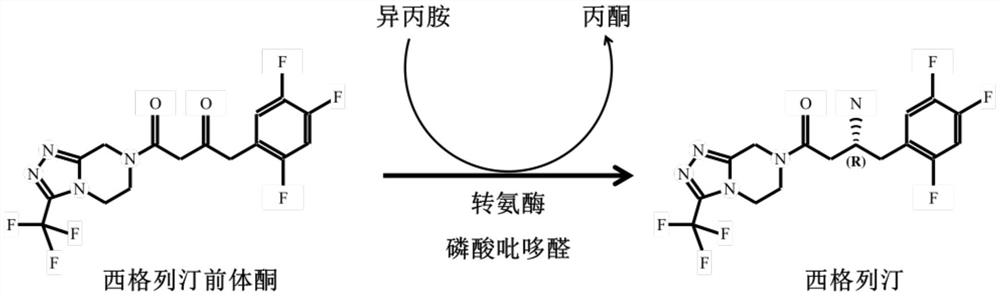
Process for preparing an intermediate of sitagliptin via enzymatic conversion
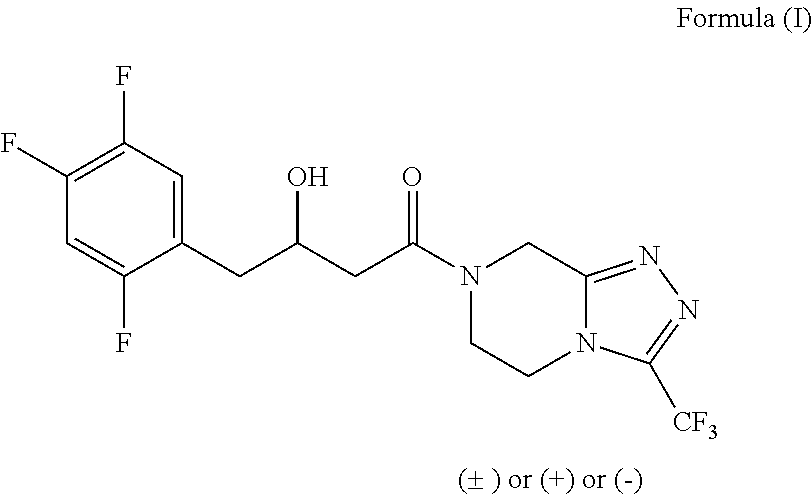
The invention provides a process for preparing 3-hydroxy-1-(3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)-4-(2,4,5-trifluorophenyl)butan-1-one (Formula I), into its racemic (R / S) form or any of its optically active (S) or (R) forms or enantiomeric excess mixture of any of the forms comprising: a) reacting 4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-2-one of formula (III) with a suitable oxidoreductase enzymes or its suitable variants in the presence of suitable conditions and co-factor; and b) isolating 3-hydroxy-1-(3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)-4-(2,4,5-trifluorophenyl)butan-1-one, into its racemic (R / S) form or any of its optically active (S) or (R) forms or enantiomeric excess mixture of any of the forms.
Owner:CADILA HEALTHCARE LTD
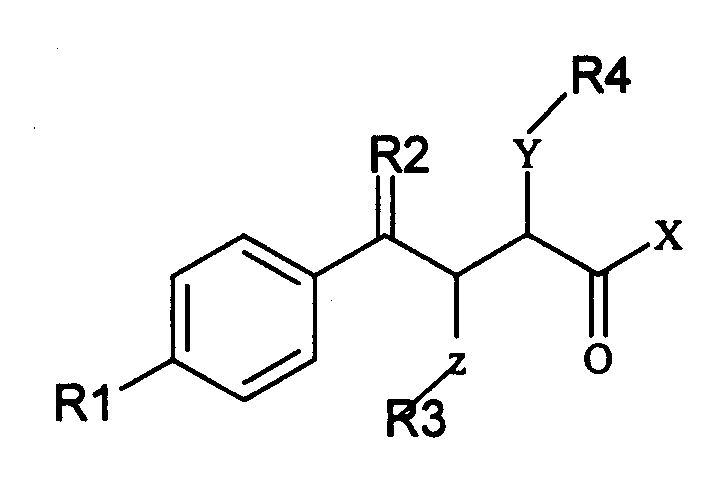
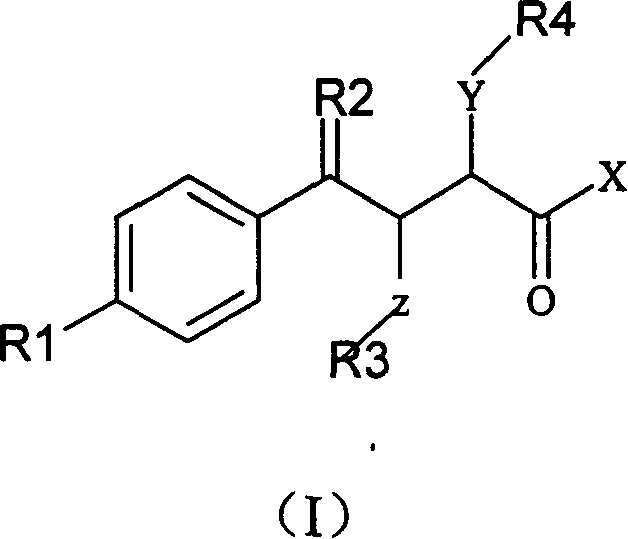
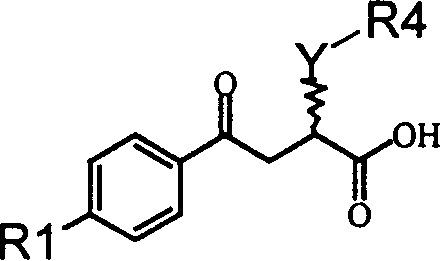
Alpha position heteroatom substituted gamma aryl ketobutyric acid derivative, process, pharmaceutical combination and uses thereof
InactiveCN1566065AActivity hasSelective inhibitionOrganic chemistrySkeletal disorderKetoneAcid derivative
The invention relates to a gamma methyl ethyl ketone acid derivative represented by general formula (I), and contains gamma-aryl-alpha-amido-beta, its preparing process, pharmaceutical compositions containing them and use as medicament, in particular as medicament for treating osteoarthritis and tumor.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
Flexible organosilicone sealant for LED package and preparation method thereof
ActiveCN105086926ASolve the problem of poor flexibilityLow modulusNon-macromolecular adhesive additivesSemiconductor devicesPolymer scienceOctanoic Acids
The present invention relates to a flexible organosilicone sealant for LED package and a preparation method thereof, and belongs to the technical field of silicone sealant. The silicone sealant includes 50-55 parts of medium-viscosity dihydroxy polydimethylsiloxane, 50-60 parts of high-viscosity dihydroxy polydimethylsiloxane, 10-16 parts of dimethyl dibutanoneoximido silane, 10-16 parts of methyltris(methylethylketoximino)silane, 20-23 parts of TiO2, 30-36 parts of mica, 0.5-2.5 parts of dibutyltin dilaurate, 0.5-0.9 part of stannous octoate and 10-18 parts of a chain extender. The present invention uses a chain extender, so that the organosilicone sealant while cross-linking increases the chain length of polymer crosslinking point molecules, and reduces the crosslinking density in network structure. The prepared sealant has low modulus and high elongation properties, and meets the requirements for such materials in LED package.
Owner:CHINA BLUESTAR CHENGRAND CO LTD
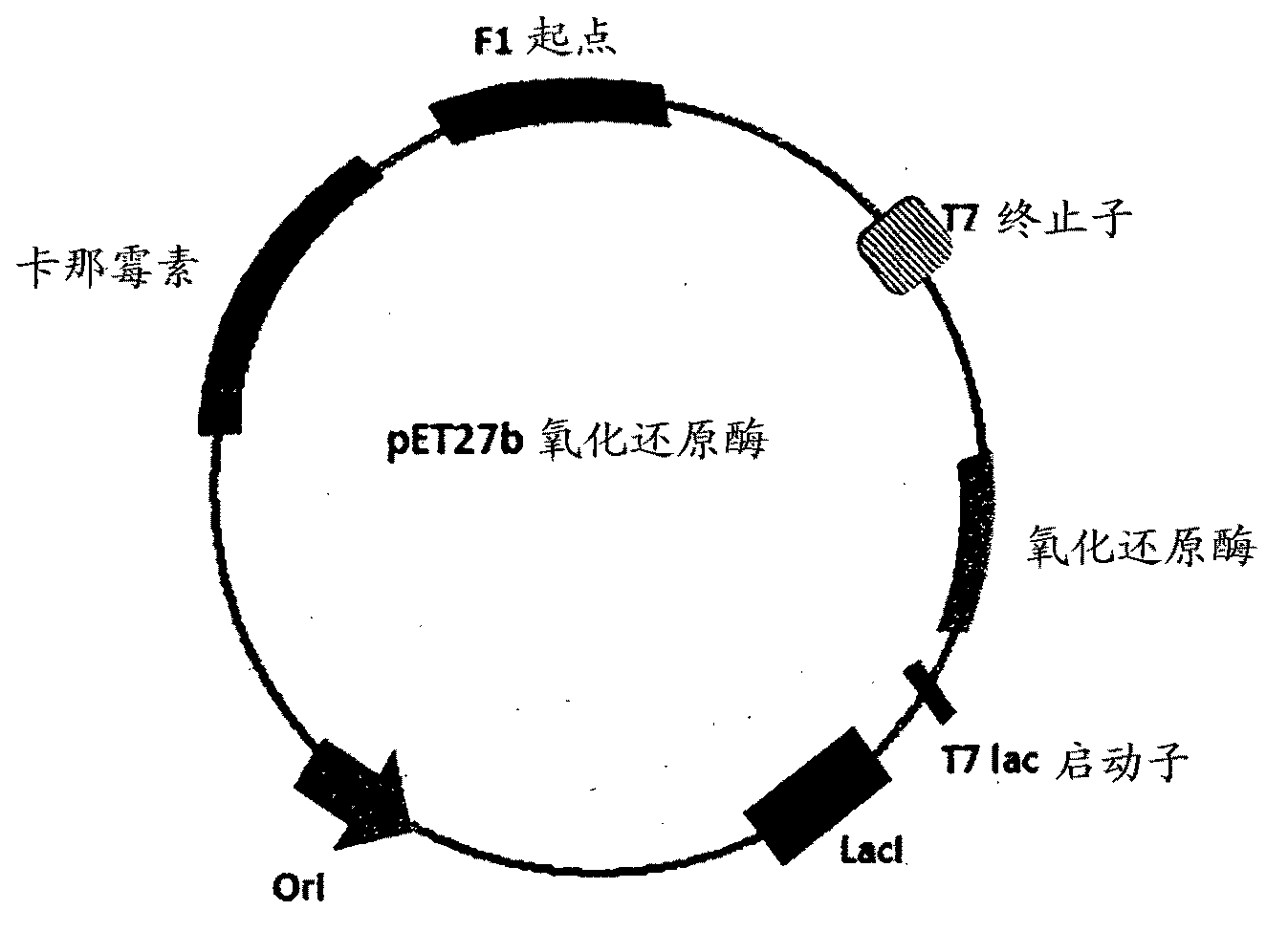
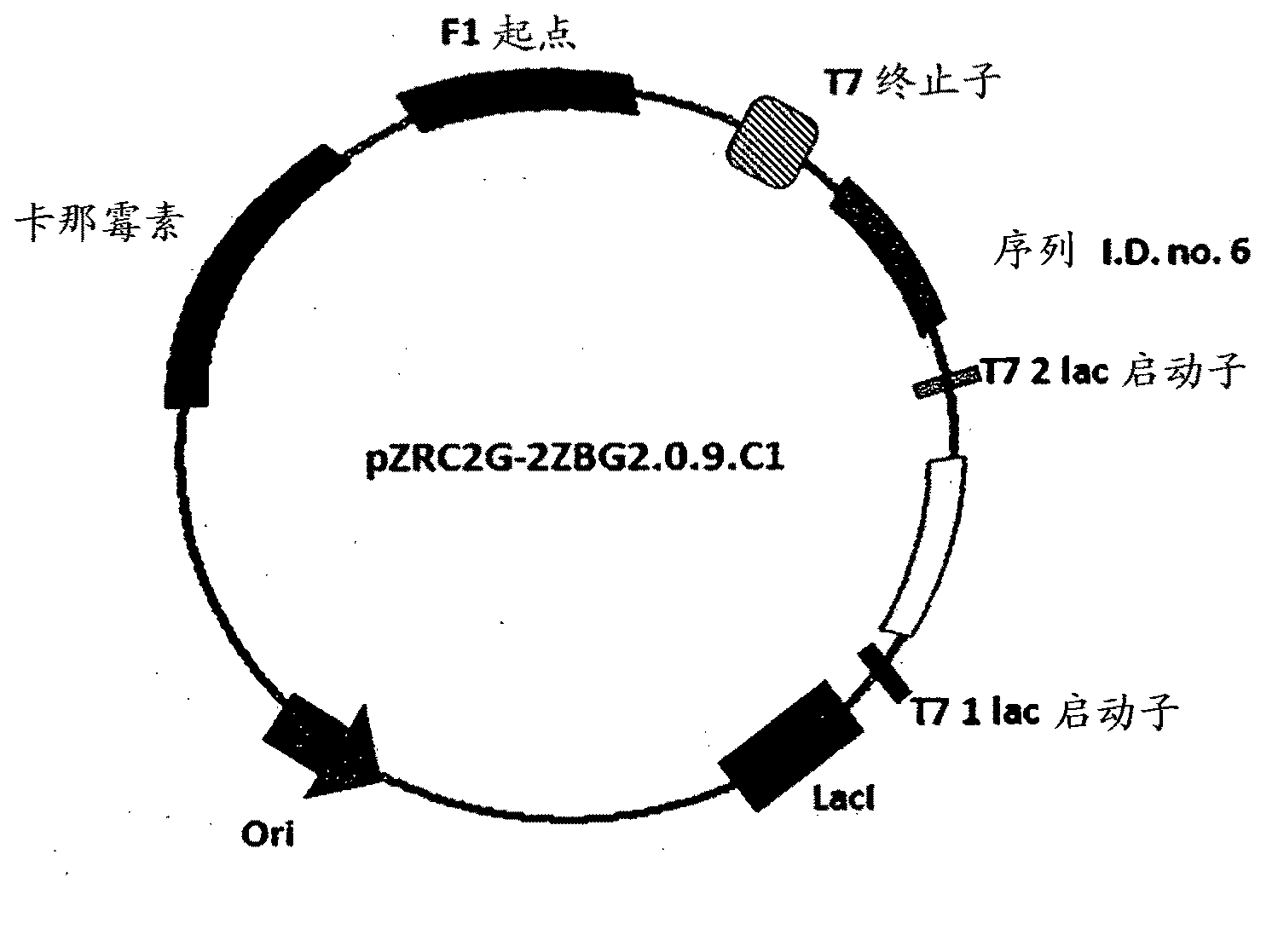
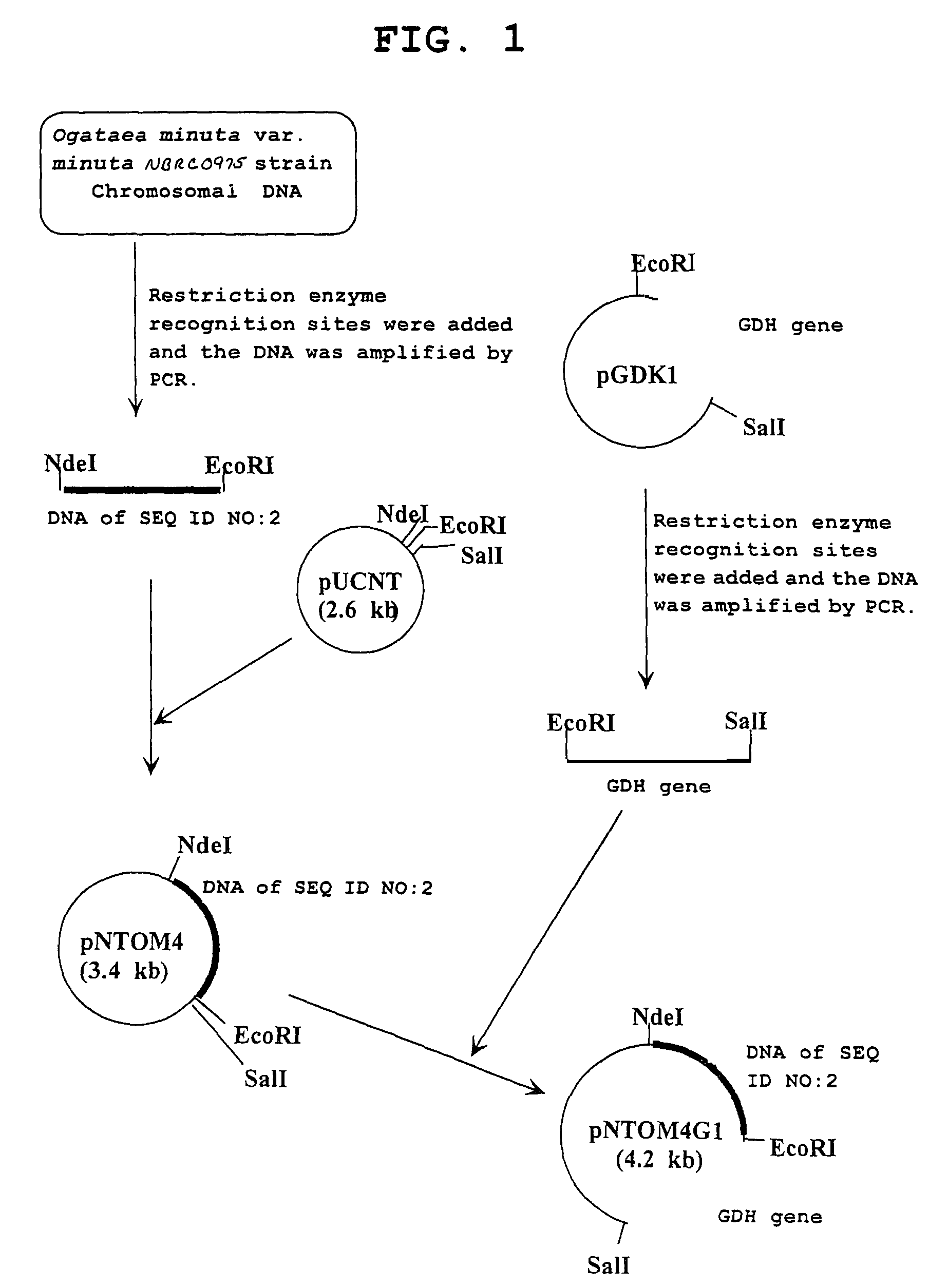
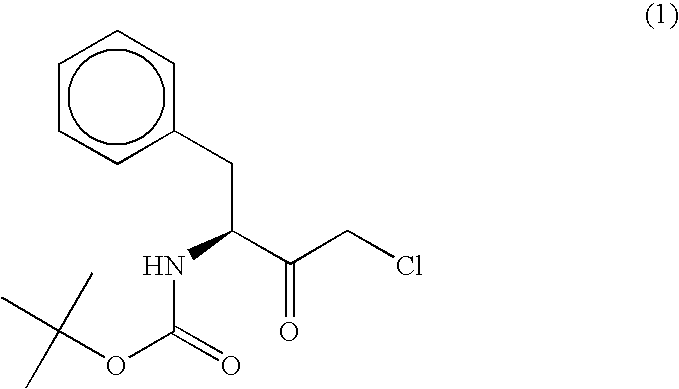
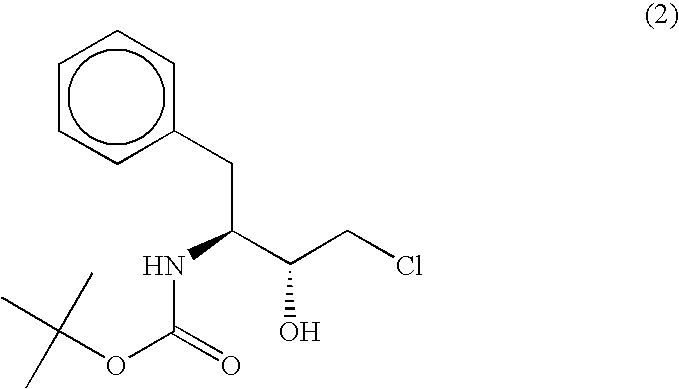
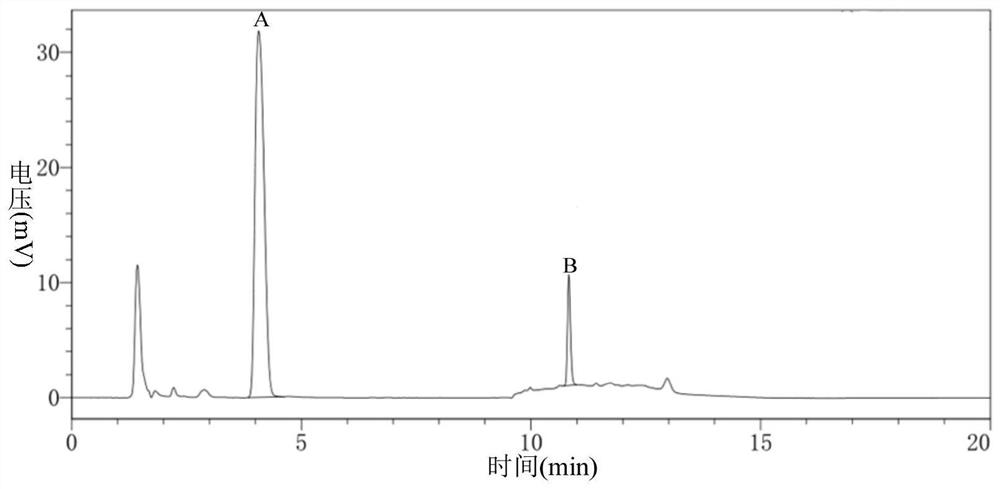
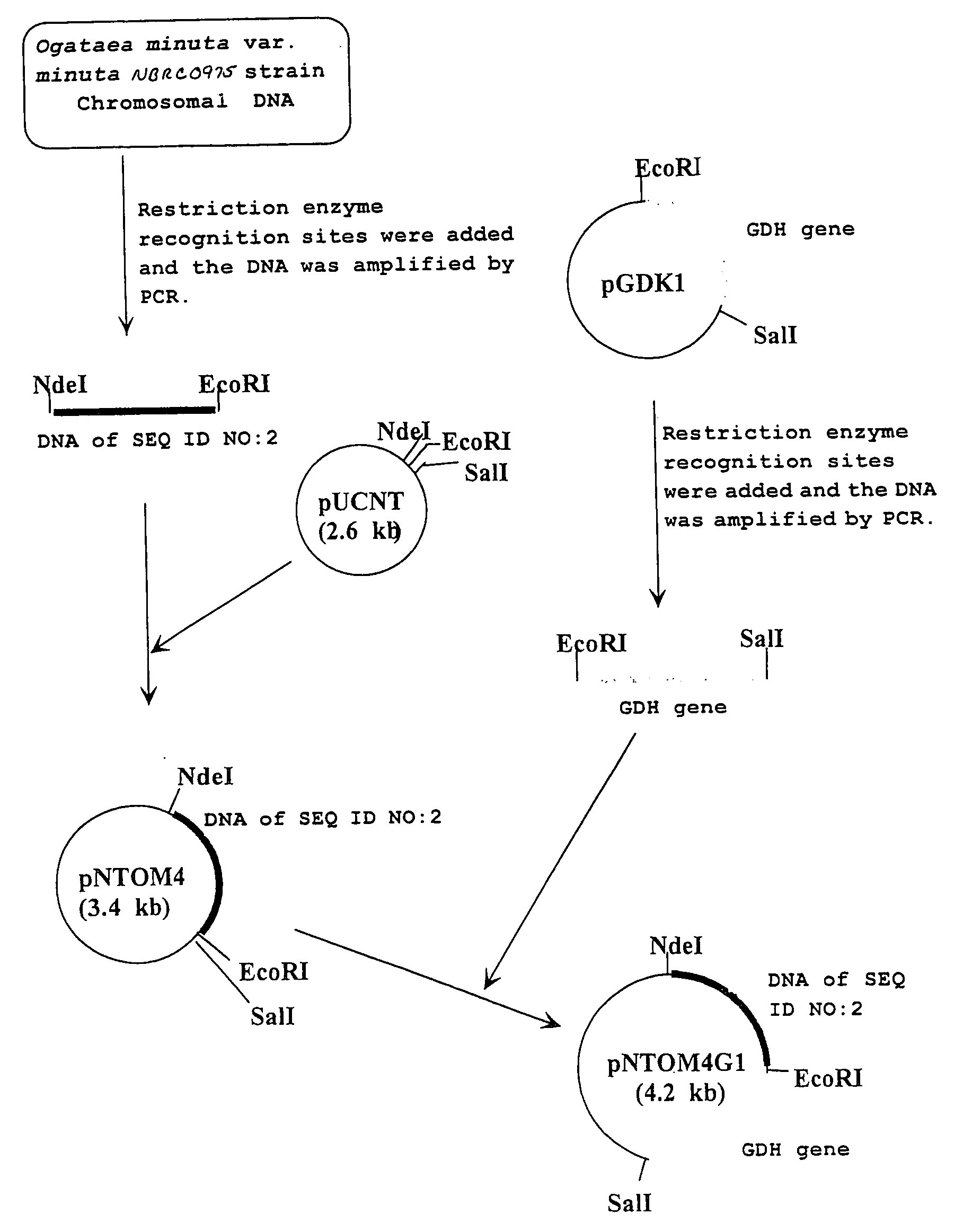
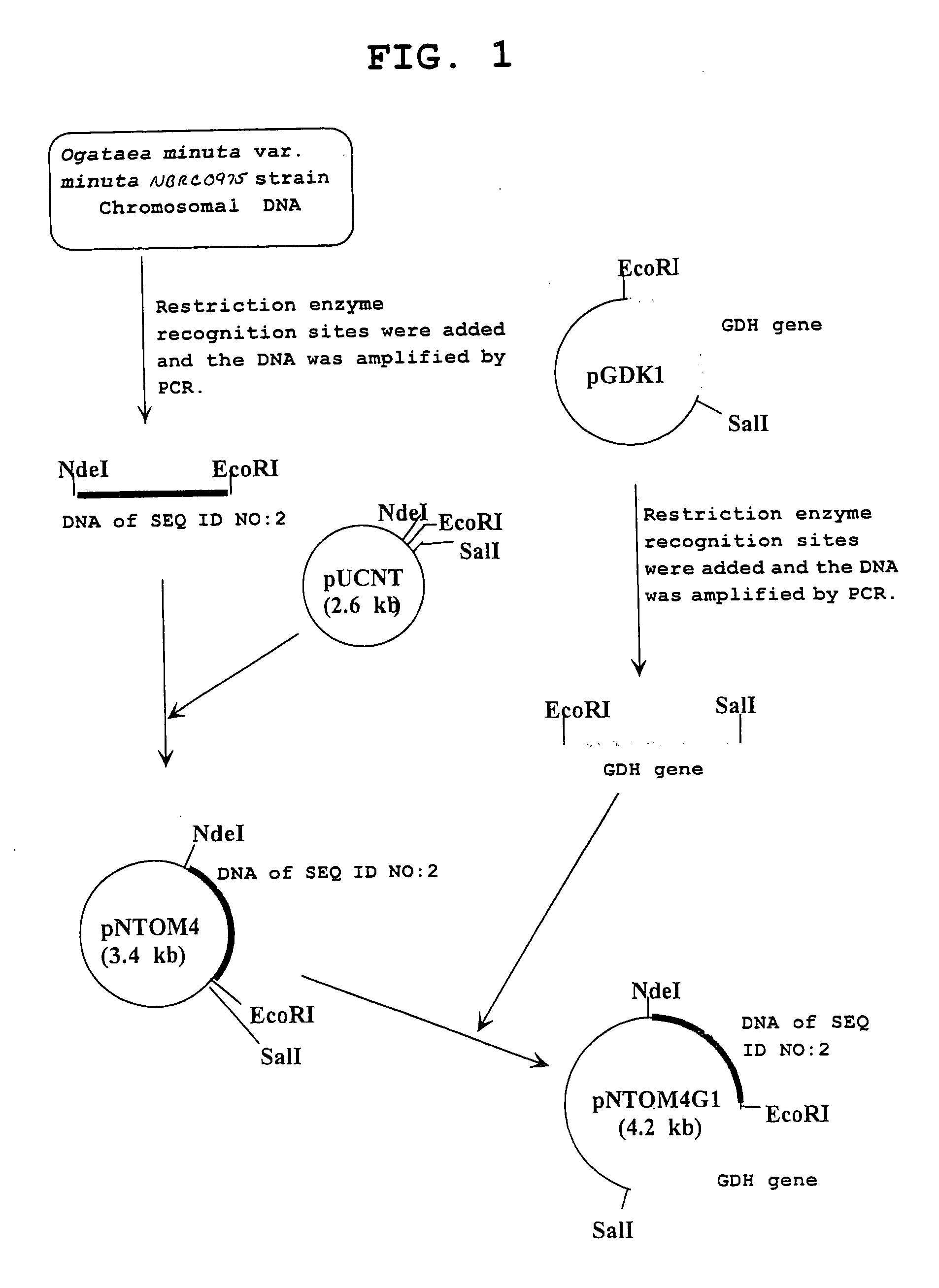
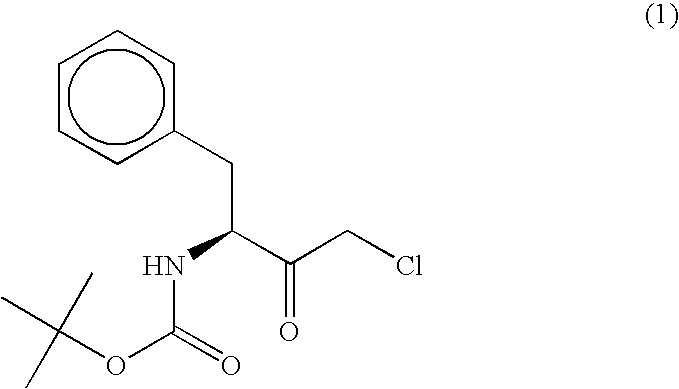
Carbonyl reductase, gene thereof and method of using the same
The present invention relates to a polypeptide having an activity to asymmetrically reduce (3S)-1-chloro-3-tert-butoxycarbonylamino-4-phenyl-2-butanone to produce (2R,3S)-1-chloro-3-tert-butoxycarbonylamino-4-phenyl-2-butanol isolated from a microorganism belonging to the genus Ogataea, a DNA encoding the polypeptide and a transformant that produces the polypeptide. The present invention moreover relates to a method of producing (2R,3S)-1-chloro-3-tert-butoxycarbonylamino-4-phenyl-2-butanol utilizing the polypeptide or the transformant. Using the polypeptide or transformant of the present invention, optically active alcohols such as (2R,3S)-1-chloro-3-tert-butoxycarbonylamino-4-phenyl-2-butanol and the like can be produced efficiently.
Owner:KANEKA CORP
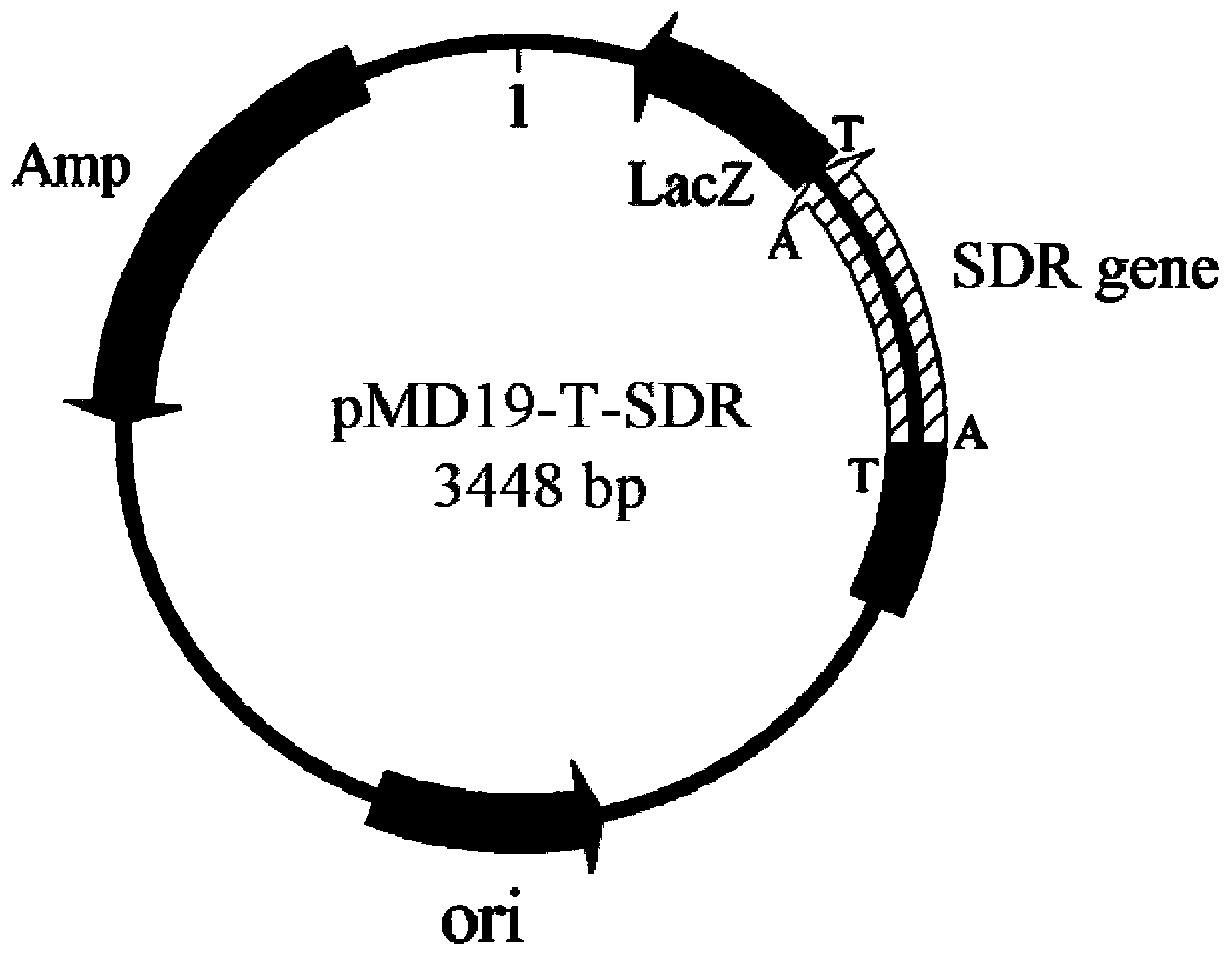
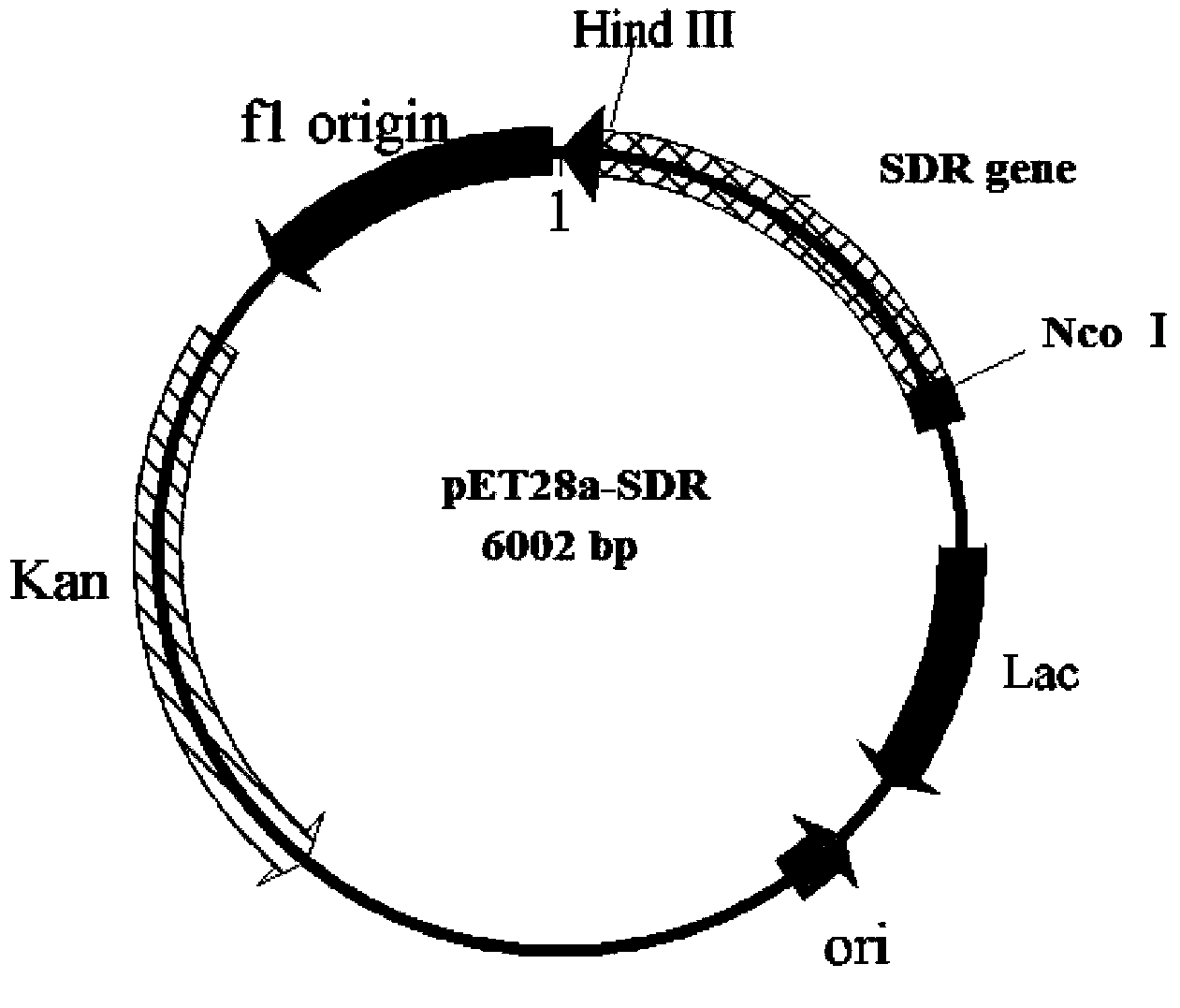
Leifsonia xyli HSO904-based short chain dehydrogenase, and encoding gene, carrier, engineering bacteria and application thereof
The invention discloses a leifsonia xyli HSO904-based short chain dehydrogenase, and an encoding gene, a carrier, engineering bacteria and application thereof. A gene of the leifsonia xyli HSO904-based short chain dehydrogenase has more than 90% of homology of a nucleotide sequence shown in SEQ ID NO. 1. A colon bacillus BL21 / pET28a (+)-SDR prepared by the recombination of the short chain dehydrogenase is used as an enzyme source, 3, 5-bis-trifluoro methyl acetophenone, trifluoromethyl acetophenone, 4-hydroxyl-2-butanone, acetoacetic ester, 4-chloro ethyl acetoacetate, acetoacetic acid tert-butyl acetate and the like are used as substrates to prepare corresponding chiral compounds such as (R)-3, 5-bis-trifluoromethyl phenethyl alcohol, trifluoromethyl benzaldehyde ethanol, 2-hydroxyl-butyl alcohol, 3-hydroxy ethyl butyrate, 4-chloro-3-hydroxy ethyl butyrate and 3-hydroxy butyric acid tert-butyl acetate through a catalytic asymmetric reduction reaction.
Owner:艾吉泰康(嘉兴)生物科技有限公司
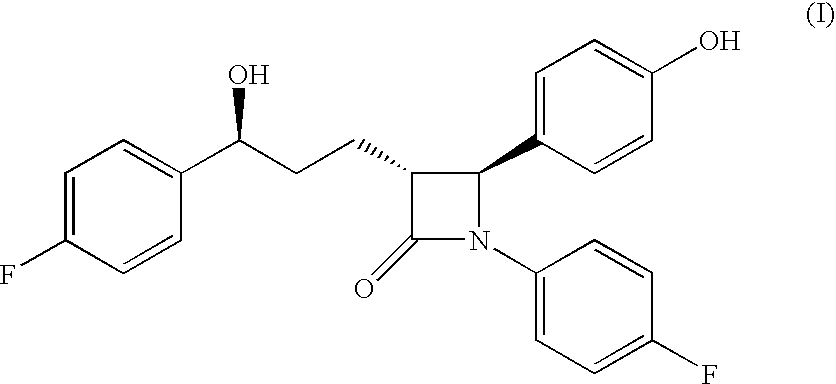
Process for the Preparation of Ezetimibe
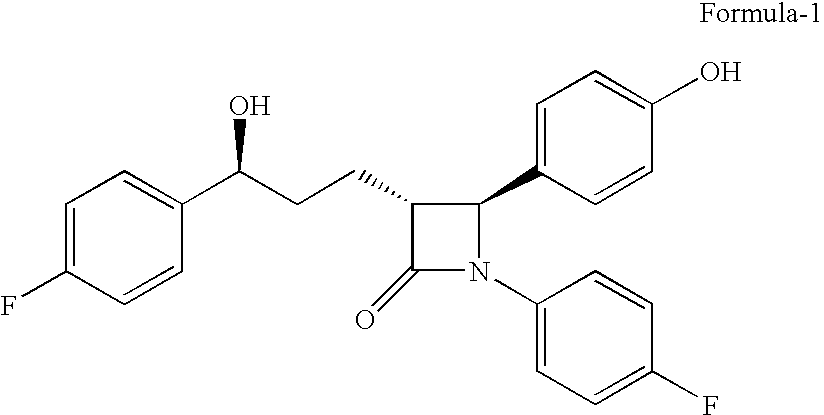
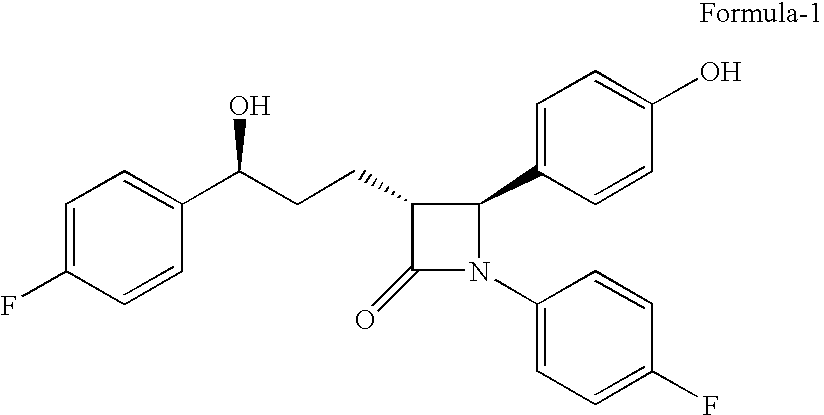
The present invention relates to a cost effective and industrially advantageous process for the preparation of (3R,4S)-1-(4-Fluorophenyl)-3-[3(S)-3-(4-fluorophenyl)-3-hydroxypropyl)]-4-(4-hydroxyphenyl)-2-azetidinone, referred to here as Ezetimibe, it is represented as formula (1).
Owner:MSN LAB PTE LTD
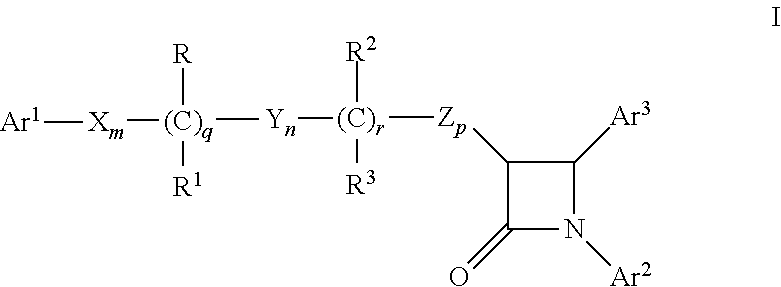
Hydroxy-substituted azetidinone compounds useful as hypocholesterolemic agents
Owner:MERCK SHARP & DOHME CORP
Process for synthesizing 4-substituted azetidinone derivatives
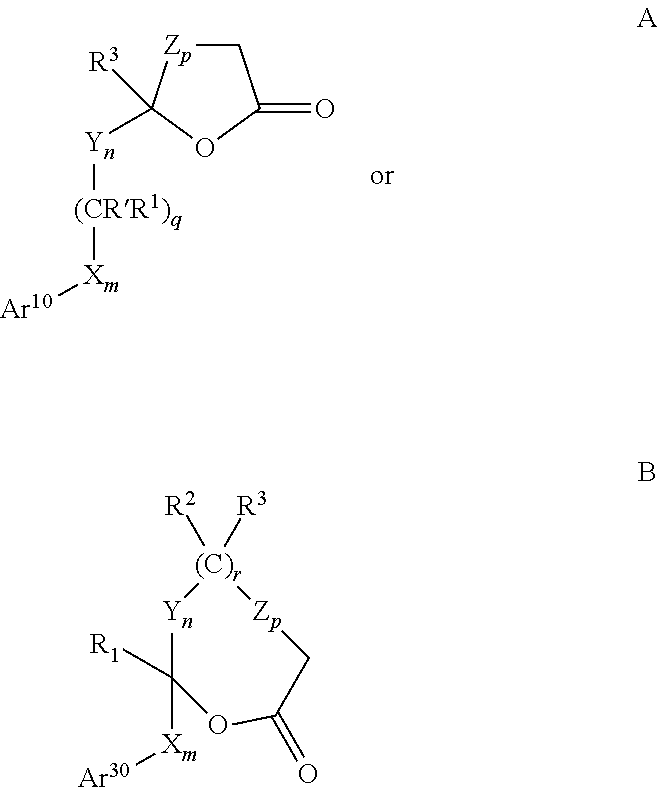
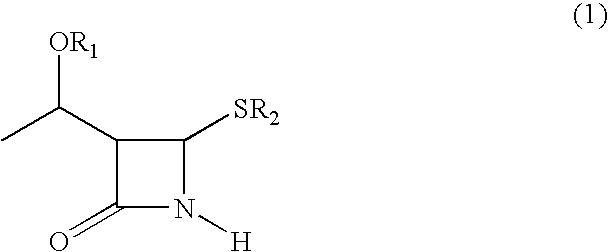
An azetidinone derivative represented by the general formula (1) (wherein OR1 is a protected hydroxyl group; R2 is a substituted or unsubstituted alkyl group, a substituted or unsubstituted alkenyl group or a substituted or unsubstituted aromatic group) is reacted with an ester compound represented by the formula (2) (wherein CO2R3 is an esterified carboxyl group; X and Y are the same or different and represent individually a substituted or unsubstituted alkyl group, a substituted or unsubstituted alkenyl group, a substituted or unsubstituted aralkyl group, a substituted or unsubstituted aryl group, a substituted or unsubstituted alkylthio group, a substituted or unsubstituted alkenylthio group, a substituted or unsubstituted aralkylthio group, a substituted or unsubstituted arylthio group, a substituted or unsubstituted alkyloxy group, a substituted or unsubstituted alkenyloxy group, a substituted or unsubstituted aralkyloxy group, a substituted or unsubstituted aryloxy group, a substituted or unsubstituted silyloxy group, a substituted or unsubstituted heterocyclic group, a substituted or unsubstituted heterocyclic-thio group, a substituted or unsubstituted heterocyclic-oxy group, a substituted or unsubstituted acyl group, a substituted or unsubstituted ester group, a substituted or unsubstituted thio ester group, a substituted or unsubstituted amide group, a substituted or unsubstituted amino group, a hydrogen atom or halogen atom, or are taken together with each other to form a substituted or unsubstituted cycloalkan-2-on-1-yl group) in the presence of zinc and copper compounds to synthesis a 4-substituted azetidinone derivative represented by the formula (3) (wherein OR1, CO2R3, X and Y are as defined above).
Owner:DAIICHI SUNTORY PHARMA CO LTD
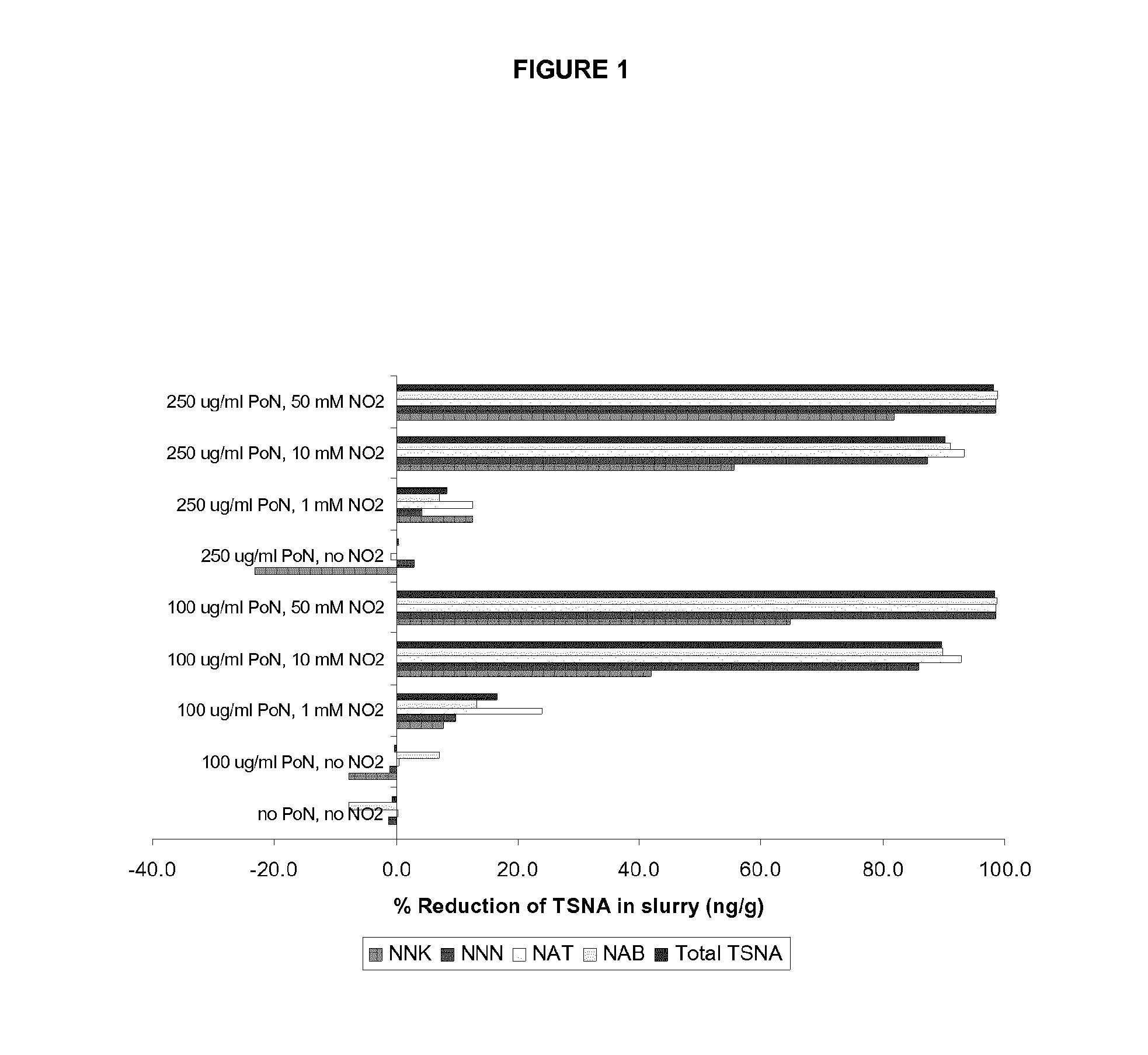
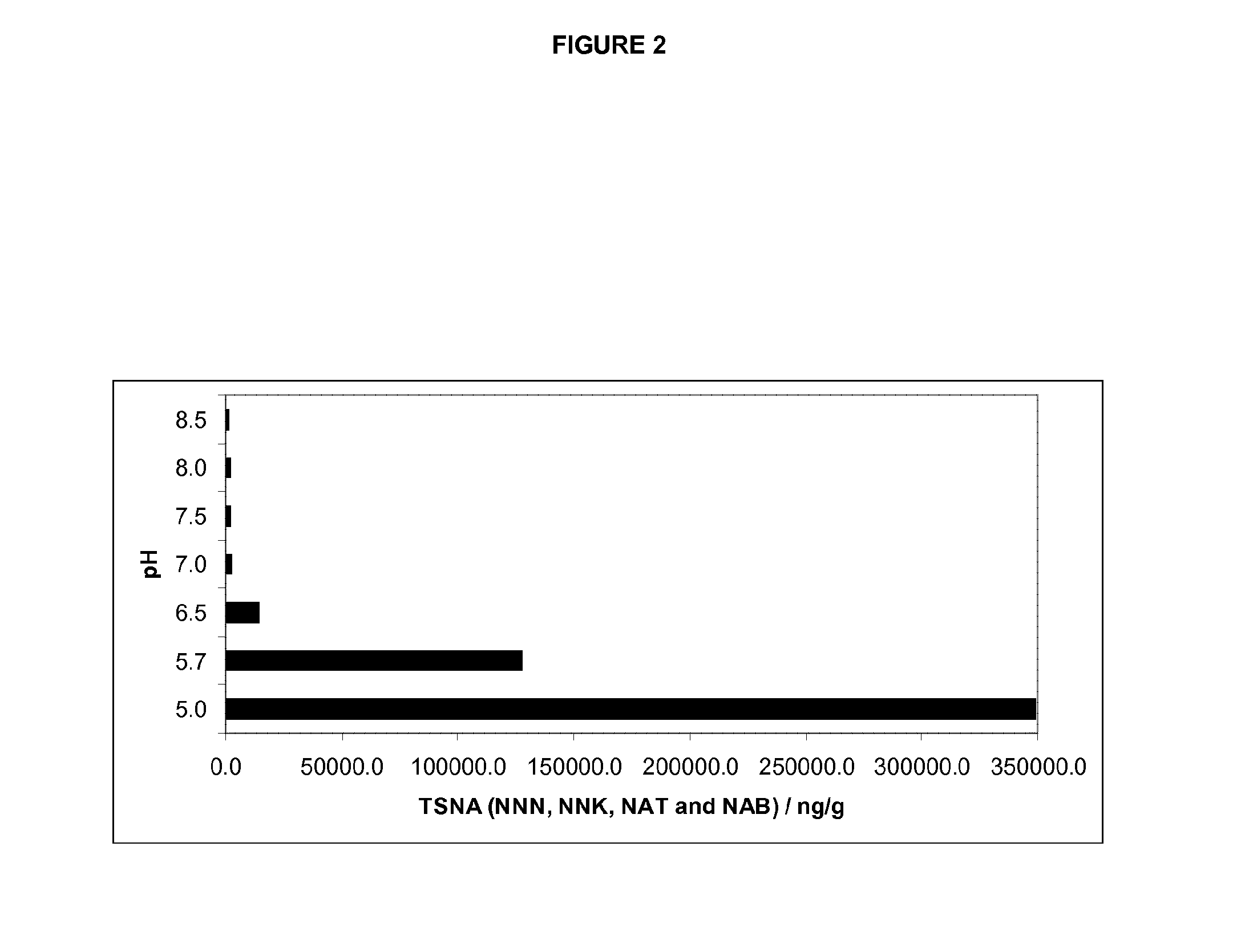
Methods for reducing the formation of tobacco specific nitrosamines in tobacco homogenates
ActiveUS20140190499A1Improve reducibilityDecrease elevated levelTobacco preparationTobacco treatmentNitrosoTobacco-specific nitrosamines
In one aspect, there is provided a method for reducing the formation of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and N-nitrosonornicotine and N-nitrosoanatabine and N-nitrosoanabasine in a tobacco homogenate comprising the steps of: (a) providing a tobacco homogenate; (b) increasing the pH of the tobacco homogenate to at least about pH 6.0; (c) optionally measuring the concentration of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and N-nitrosonornicotine and N-nitrosoanatabine and N-nitrosoanabasine in the tobacco homogenate before and after the pH treatment in step (b); and (d) obtaining a tobacco homogenate in which the levels of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and N-nitrosonornicotine and N-nitrosoanatabine and N-nitrosoanabasine are reduced as compared to the levels of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and N-nitrosonornicotine and N-nitrosoanatabine and N-nitrosoanabasine in the tobacco homogenate provided in step (a).
Owner:PHILIP MORRIS PROD SA
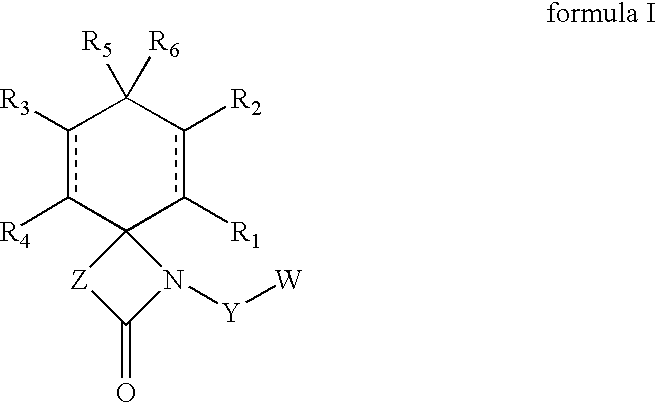
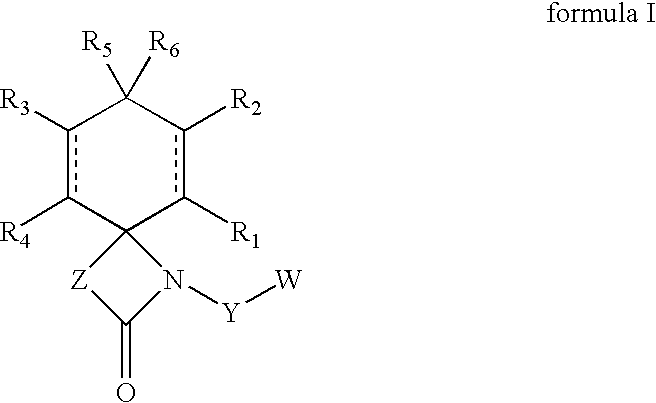
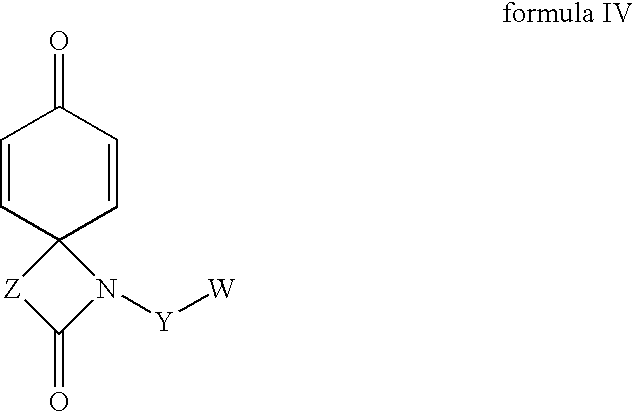
Regioselective hydroxylation, functionalisation and protection of spirolactams
The present invention refers to highly functionalised spiro-fused azetidinones of formula I:having a cyclohexane moiety with the desired number of protected or unprotected hydroxyl groups which are introduced with high stereo and regioselectivity, as well as processes for their synthesis.
Owner:LAB DEL DR ESTEVE SA
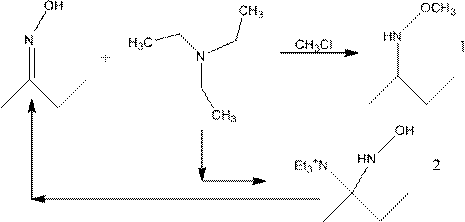
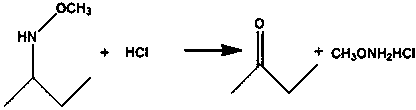
Preparation method of methoxylamine hydrochloride
The invention provides a preparation method of methoxylamine hydrochloride. The method comprises the following steps of adding diacetylmonoxime (C4H9NO), dimethyl sulfoxide (DMSO, C2H6OS), triethylamine (C6H15N) and a methylation reagent into a reaction vessel, and reacting at 15-75 DEG C to generate O-methyl-2-diacetylmonoxime ether. Compared with the prior art. The method has the advantages thatthe operation is simple, wastes are few, furthermore, reaction raw materials can be completely converted, a generated intermediate by-product can be decomposed into diacetylmonoxime (C4H9NO) and triethylamine (C6H15N), equivalently, no side reaction exists, the yield of synthesized methoxylamine hydrochloride is improved, the use of toxic substances such as sulfur dioxide and sodium nitrite is avoided, the emission of toxic gases such as nitric oxide is reduced, and the sustainable development of enterprises is facilitated.
Owner:SHANDONG JINCHENG KERUI CHEMICAL CO LTD
Transaminase mutant and applications of transaminase mutant in Sitagliptin synthesis
ActiveCN112094856AHigh stereoselectivityIncrease enzyme activityBacteriaTransferasesSitagliptinPyrazine
The invention discloses a mutant of transaminase SEQ ID NO: 1. The mutant has significantly enhanced enzyme activity and high stereoselectivity; the mutant can efficiently catalyze (2Z)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazine-7-(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-2-one to synthesize the Sitagliptin, and the e.e. value of products is greater than 99.95%, so thatthe mutant has industrial application prospects.
Owner:SHANXI WEIQIDA PHARMA IND
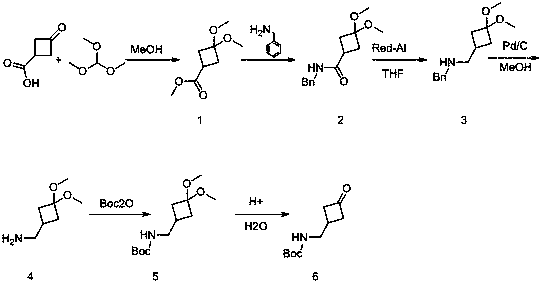
Method for synthesizing 3-(Boc-aminomethyl)cyclobutanone
ActiveCN109053496ALow costUnique routeCarbamic acid derivatives preparationOrganic compound preparationSodium methoxideSynthesis methods
The invention relates to a method for synthesizing 3-(Boc-aminomethyl)cyclobutanone. The method for synthesizing 3-(Boc-aminomethyl)cyclobutanone provided by the invention mainly solves the technicalproblems of high raw material cost and relatively difficult post-processing according to the existing synthesis method. The method for synthesizing 3-(Boc-aminomethyl)cyclobutanone provided by the invention comprises the following steps: 3-oxocyclobutanecarboxylic acid and trimethyl orthoformate are reacted in the methanol solution to generate a compound 1; the compound 1 and benzylamine are reacted in the methanol solution under an action of sodium methoxide to generate a compound 2; the compound 2 and sodium bis(2-methoxyethoxy)aluminiumhydride are reacted in tetrahydrofuran solution to generate a compound 3; the compound 3 is subjected to a Pd / C debenzylation and hydrogenation operation in the methanol solution to obtain a compound 4; the compound 4 and Boc2O are reacted in the methanolsolution to obtain a compound 5; the compound 5 is reacted in a 0.05 M hydrochloric acid solution generate a target compound 6. By performing an enzymatic catalyzed deglandulation action of on the corresponding prochiral 3-substituted cyclobutanone, a series of gamma-butyrolactone derivatives, including some spiro derivatives, can be obtained; and all the derivatives are inhibitors having good biological activity.
Owner:GL BIOCHEM SHANGHAI
Process for preparing an intermediate of sitagliptin via enzymatic conversion
The invention provides a process for preparing 3-hydroxy-1-(3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)-4-(2,4,5-trifluorophenyl)butan-1-one (Formula I), into its racemic (R / S) form or any of its optically active (S) or (R) forms or enantiomeric excess mixture of any of the forms comprising:a) reacting 4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-2-one of formula (III) with a suitable oxidoreductase enzymes or its suitable variants in the presence of suitable conditions and co-factor; andb) isolating 3-hydroxy-1-(3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)-4-(2,4,5-trifluorophenyl)butan-1-one, into its racemic (R / S) form or any of its optically active (S) or (R) forms or enantiomeric excess mixture of any of the forms.
Owner:CADILA HEALTHCARE LTD
(R)-omega-transaminase mutant and application thereof in preparation of sitagliptin intermediate
ActiveCN111534494AIncrease enzyme activityIncreased substrate toleranceBacteriaTransferasesArginineThreonine
The invention discloses a (R)-omega-transaminase mutant and an application thereof in preparation of a sitagliptin intermediate. The mutant is obtained through multipoint mutation of arginine at the 77th site, leucine at the 181st site, arginine at the 130th site, tyrosine at the 139th site and threonine at the 273th site of an amino acid sequence shown as SEQ ID NO.1. According to the invention,a novel (R)-omega-TA recombinase is screened through a gene mining technology; molecular modification is carried out through a protein engineering technology; an (R)-omega-TA mutant catalyst with highenzyme activity, high substrate tolerance and high stereoselectivity is obtained; and the mutant can be used for asymmetric catalytic synthesis of the sitagliptin intermediate (R)-3-amino-1-(pyrrolidine-1-yl)-4-(2, 4, 5-trifluorophenyl) butan-1-one by taking a precursor ketone analogue 1-(pyrrolidine-1-yl)-4-(2, 4, 5-trifluorophenyl)-1, 3-butanedione as a substrate, and has higher conversion rate.
Owner:ZHEJIANG UNIV OF TECH +2
Synthesis method of trans-4-oxo-2-hexenal
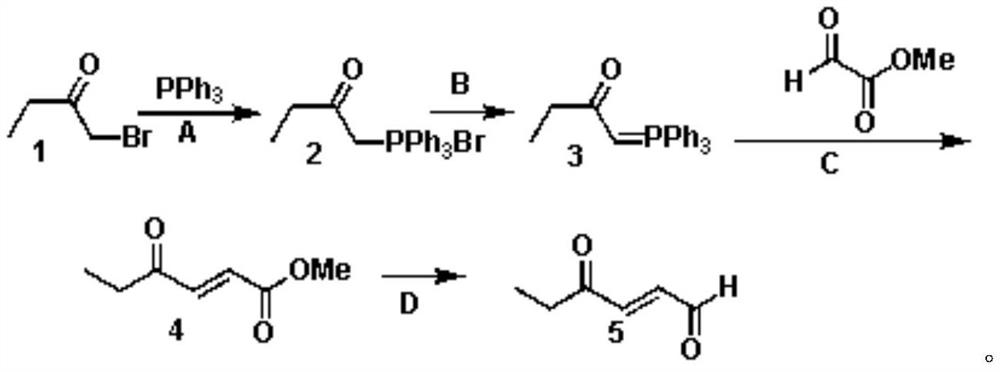
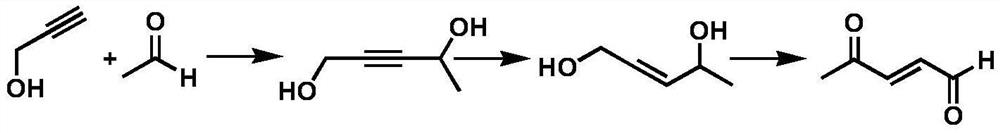
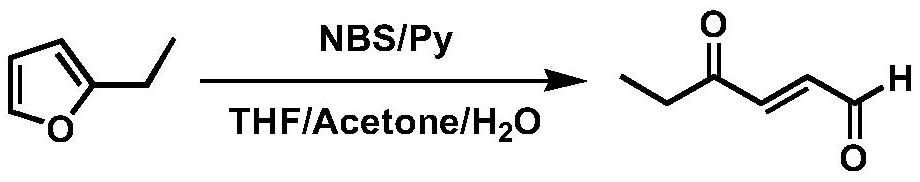
PendingCN111747837ALow costEasy to operateOrganic compound preparationGroup 5/15 element organic compoundsButyloneHexene
Owner:SUZHOU HUADAO BIOLOGICAL PHARMA
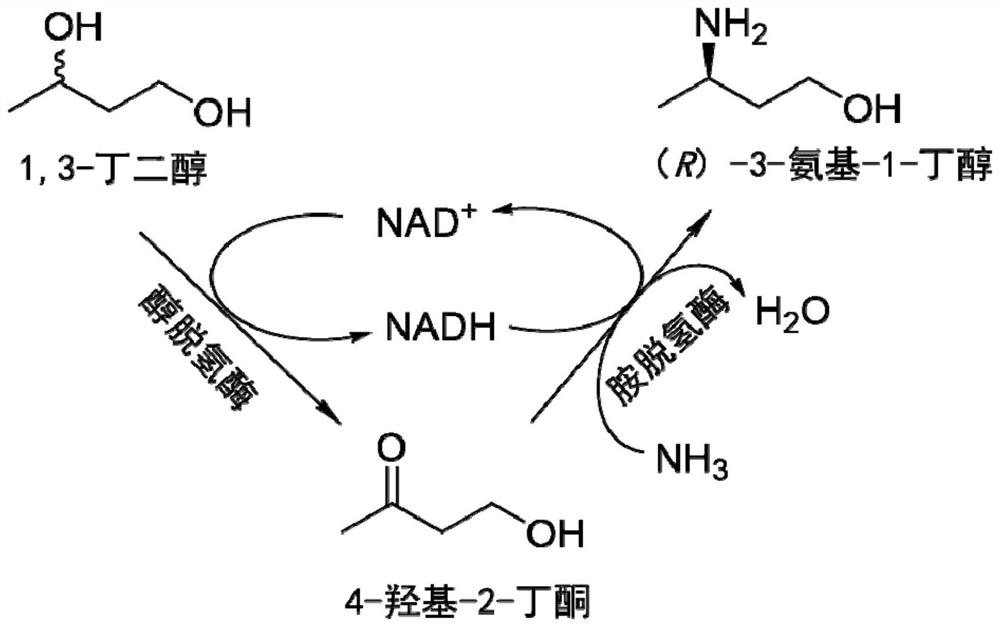
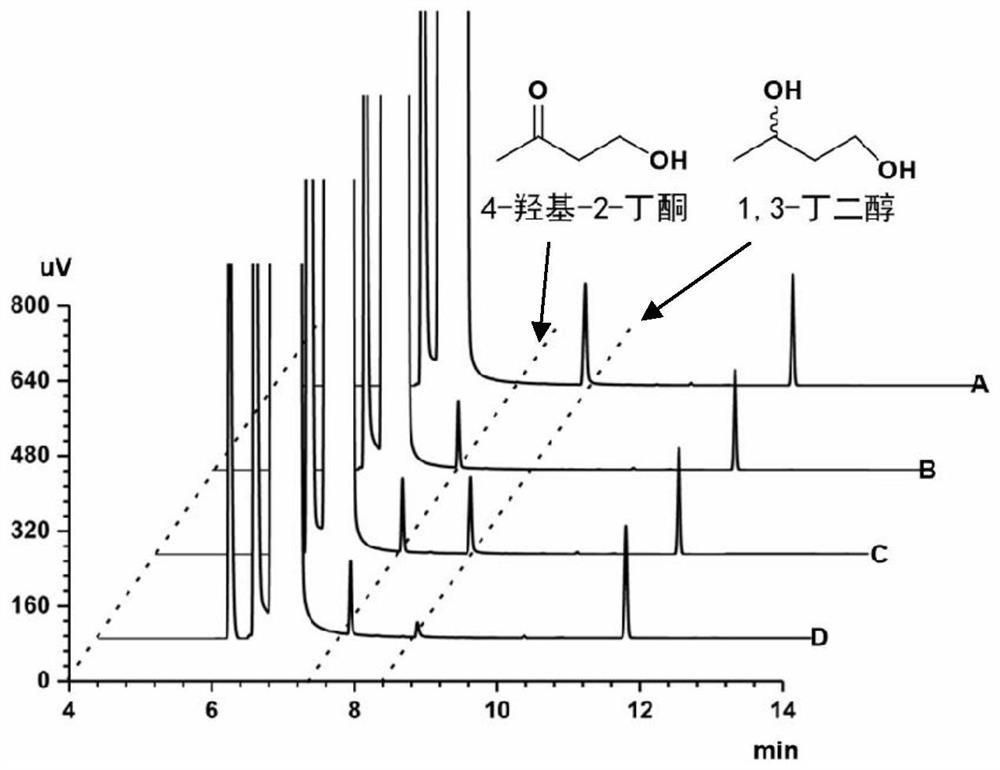
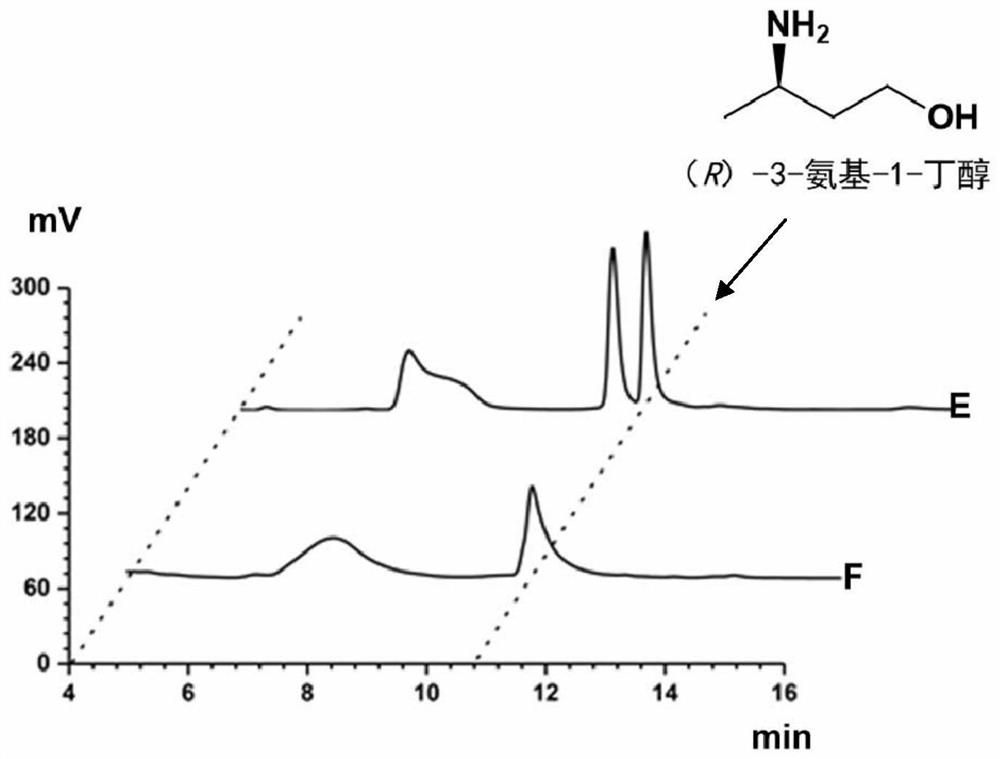
Method for synthesizing (R)-3-amino-1-butanol through double-enzyme cascade catalysis
The invention discloses a method for synthesizing (R)-3-amino-1-butanol through double enzyme cascade catalysis. The method comprises the following steps: with 1, 3-butanediol as a substrate, carrying out a catalytic reaction with alcohol dehydrogenase to generate 4-hydroxy-2-butanone; and taking 4-hydroxy-2-butanone as a substrate, and generating chiral (R)-3-amino-1-butanol through a catalytic reaction of amine dehydrogenase or a mutant of the amine dehydrogenase. The invention provides a brand-new green biosynthesis route, and the chiral (R)-3-amino-1-butanol is catalytically synthesized by using cheap 1, 3-butanediol as a raw material through double-enzyme cell co-expression. Meanwhile, the method provided by the invention has a cofactor self-circulation system and has good economic benefits. The method has an important application value.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
Environment-friendly plastic material
InactiveCN106243698AImprove flame retardant performanceHigh smoke suppression effectCellulosePolytetramethylene terephthalate
The invention discloses an environment-friendly plastic material. The material is prepared from, by weight, 50-120 parts of nylon, 42-78 parts of cis-hexenyl dicarboxylic anhydride-allyl benzene copolymer, 35-65 parts of 2-hydroxyl-4-octyl oxyl phenylbutyrophenone, 20-38 parts of butenyl distearamide, 15-30 parts of polybutylene terephthalate, 12-25 parts of polyether glycerol, 10-20 parts of brominated paraffin, 10-15 parts of olive oil, 8-14 parts of AR glass fiber, 6-12 parts of carboxyethyl cellulose, 15-25 parts of filler, 8-12 parts of addition agent, 4-6 parts of fire retardant, 3-5 parts of antismoke agent, 1-3 parts of ultraviolet light absorber, 1-3 parts of tackifier and 1-2 parts of softening agent. The environment-friendly plastic material is good in flame retardant effect, high in anti-impact capacity, high in toughness and good in performance, is low in preparation cost, can be applied and popularized and has remarkable economic and social benefits.
Owner:冯可发
3-hydroxy-2-butanone-2,4-dinitrophenylhydrazone and method for detecting acetoin content in exhaled breath by using same
PendingCN111732523AThe reaction conditions are simple and not harshShort processComponent separationHydrazone preparationPhysical chemistryBiology
The invention provides 3-hydroxyl-2-butanone-2,4-dinitrophenylhydrazone and a method for detecting acetoin content in exhaled breath by using the 3-hydroxyl-2-butanone-2,4-dinitrophenylhydrazone, andbelongs to the field of biochemical engineering. The invention relates to 3-hydroxy-2-butanone-2,4-dinitrophenylhydrazone, of which the structural formula of the compound is shown as a formula (I), and the 3-hydroxy-2-butanone-2,4-dinitrophenylhydrazone is a new compound and can be used for detecting the acetoin content in exhaled breath of a human body, so that a new application field is developed.
Owner:必睿思(杭州)科技有限公司
Preparation method of sitagliptin intermediate
ActiveCN113481254AHigh recovery rateEasy to separate and purifyChemical recyclingFermentationPyrazineOrganosolv
The invention discloses a preparation method of a sitagliptin intermediate and the sitagliptin intermediate prepared by the preparation method, the preparation method comprises the step that in the presence of an organic solvent with a boiling point not higher than 110 DEG C at a standard atmospheric pressure, carrying out transamino contact between transaminase and a substrate sitagliptin precursor ketone (2Z)-4-oxo-4-[3-(trifluoromethyl)-5, 6-dihydro-[1, 2, 4]triazolo[4, 3-a]pyrazine-7-(8H)-yl]-1-(2, 4, 5-trifluorophenyl) butyl-2-one to perform enzyme catalysis reaction so as to prepare the sitagliptin intermediate (3R)-3-amino-1-[3-(trifluoromethyl)-5, 6, 7, 8-tetrahydro-1, 2, 4-triazolo [4, 3-a] pyrazine-7-yl]-4-(2, 4, 5-trifluorophenyl) butyl-1-one, a mixed solution obtained by mixing a low-boiling-point organic solvent and the substrate is added into a premixing system containing the transaminase in a fed-batch mode, ketoreductase and a coenzyme regeneration system in a specific proportion are added into the premixing system, the substrate conversion rate is increased, and the substrate conversion rate can reach 99%.
Owner:台州酶易生物技术有限公司
Novel Carbonyl Reductase, Gene Thereof and Method of Using the Same
The present invention relates to a polypeptide having an activity to asymmetrically reduce (3S)-1-chloro-3-tert-butoxycarbonylamino-4-phenyl-2-butanone to produce (2R,3S)-1-chloro-3-tert-butoxycarbonylamino-4-phenyl-2-butanol isolated from a microorganism belonging to the genus Ogataea, a DNA encoding the polypeptide and a transformant that produces the polypeptide. The present invention moreover relates to a method of producing (2R,3S)-1-chloro-3-tert-butoxycarbonylamino-4-phenyl-2-butanol utilizing the polypeptide or the transformant.Using the polypeptide or transformant of the present invention, optically active alcohols such as (2R,3S)-1-chloro-3-tert-butoxycarbonylamino-4-phenyl-2-butanol and the like can be produced efficiently.
Owner:KANEKA CORP
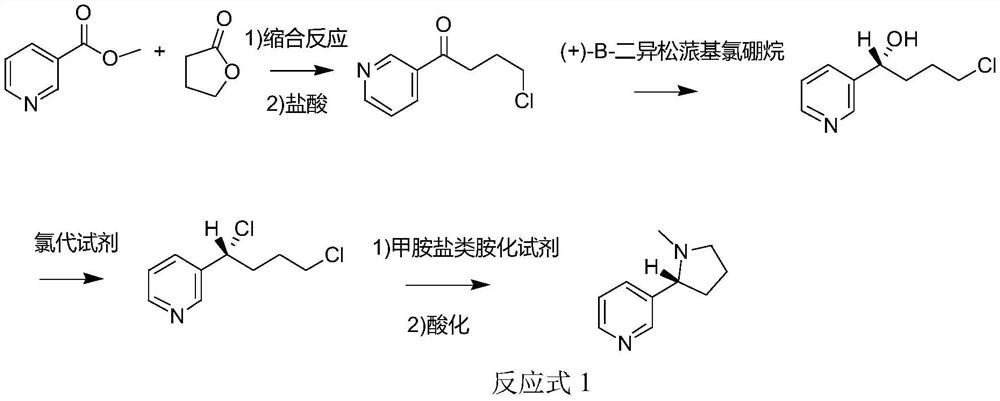
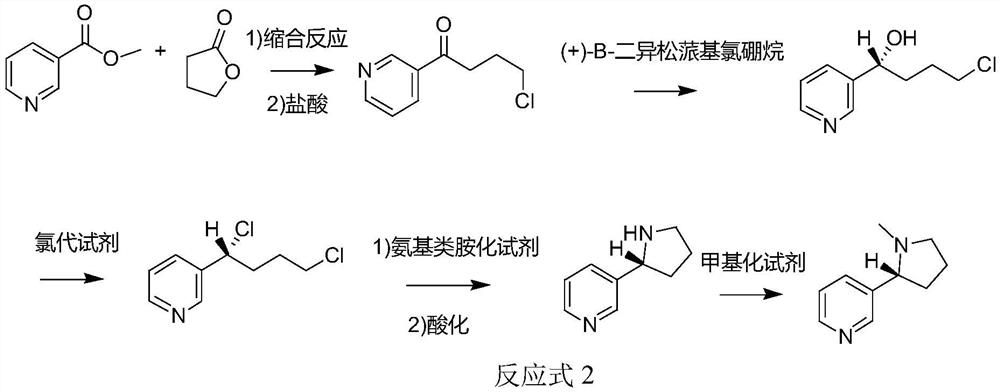
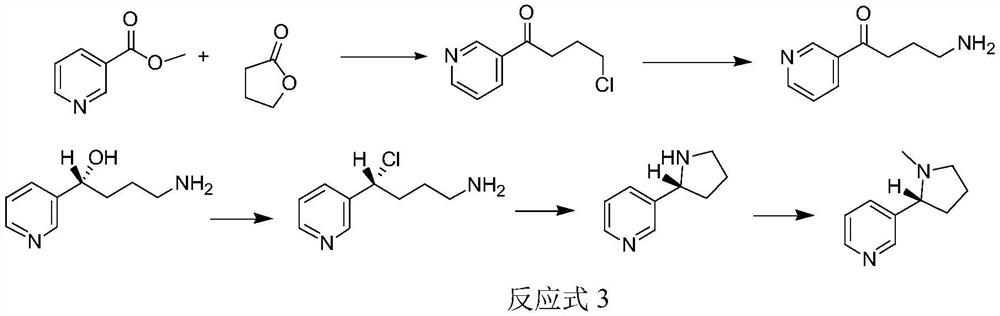
Method for synthesizing chiral nicotine from butyrolactone
ActiveCN113582972AHigh yieldHigh purityOrganic chemistryBulk chemical productionNicotinuric acidMethyl palmoxirate
The invention discloses a method for synthesizing chiral nicotine from butyrolactone, which comprises the following steps: condensing nicotinate and gamma-butyrolactone under the action of a basic catalyst, carrying out reflux reaction with hydrochloric acid to obtain 4-chloro-1-(3-pyridine)-1-butanone, inducing the 4-chloro-1-(3-pyridine)-1-butanone by (+)-B-diisopinocampheyl borane to generate chiral hydroxyl so as to obtain (S)-4-chloro-1-(pyridine-3-yl) butyl-1-ol, reacting the material with a chlorination reagent to generate (S)-3-(1, 4-dichlorobutyl) pyridine, carrying out a ring closing reaction on (S)-3-(1, 4-dichlorobutyl) pyridine and an amination reagent under alkaline conditions to obtain (S)-demethylated nicotine or (S)-nicotine, wherein methylation is carried out on (S)-demethylated nicotine to obtain (S)-nicotine. Whether methylation reaction is needed or not can be determined according to the type of the amination reagent, and the yield of the prepared (S)-nicotine is high.
Owner:SHENZHEN ZINWI BIO-TECH CO LTD
Synthesis of optically-pure sulfur-containing quaternary heterocyclic drug intermediate
ActiveCN108358887AHigh optical purityHigh yieldOrganic chemistry methodsFermentationAcetic acidTert-Butyloxycarbonyl protecting group
The invention provides a novel method for synthesizing an optically-pure sulfur-containing quaternary heterocyclic drug intermediate which is (S)-2-[(tert-butyloxycarboryl)-amino]-2-[3-(3-methoxy)-1-cyclopropylsulfonyl]-acetic acid. The method comprises the following steps: taking thietanone as a starting raw material, carrying out Wittig-horner reaction, methoxylation, oxidization and enzymatic catalysis hydrolytic resolution to prepare an optically-pure target compound. The method is easily available in raw materials, simple in requirements on equipment and efficient in synthesis of chiral compounds; the steps can be enlarged to kilogram scale for production; the method is a novel method suitable for industrial application.
Owner:富乐马鸿凯(大连)医药有限公司
Transaminase and application thereof in preparation of sitagliptin
The invention discloses transaminase and application thereof in preparation of sitagliptin. The amino acid sequence of the transaminase is shown as SEQ ID NO: 1, or the amino acid sequence of the transaminase is at least 80%, at least 85%, at least 90%, at least 95%, at least 96%, at least 97%, at least 98% or at least 99% similar to the SEQ ID NO: 1. The transaminase can asymmetrically catalyze sitagliptin precursor ketone (2Z)-4-oxo-4-[3- (trifluoromethyl)-5, 6-dihydro-[1, 2, 4] triazolo [4, 3-a] pyrazine-7-(8H)-yl]-1-(2, 4, 5-trifluorophenyl) butyl-2-ketone to generate sitagliptin intermediate (3R)-3-amino-1-[3-(trifluoromethyl)-5, 6, 7, 8-tetrahydro-1, 2, 4-triazolo [4, 3-a] pyrazine-7-yl]-4- (2, 4, 5-trifluorophenyl) butyl-1-ketone, the enzyme activity of the transaminase can reach 1100U / g, and when an enzymatic reaction is carried out for 2h, 50 microliters of transaminase liquid can make the substrate conversion rate reach 55%. Compared with existing transaminase for catalyzing the same reaction, the transaminase has the advantages of high activity, ideal stability and good organic solvent tolerance.
Owner:台州酶易生物技术有限公司
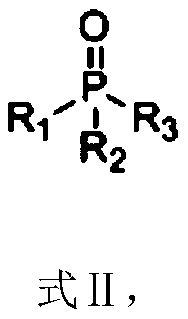
Composition containing trisubstituted phosphine oxide and oxetanone derivative and application thereof
The invention provides a composition containing a trisubstituted phosphine oxide and an oxetanone derivative. The specific definitions of the trisubstituted phosphine oxide and the oxetanone derivative are shown in the specification. The composition provided by the invention can generate the synergistic bacteriostatic effect (combined medication index CI is less than 1)) on various bacteria such as corynebacterium acidovorans and the like.
Owner:ZHONGSHAN WANHAN PHARM CO LTD
Refining method of methyltris (methylethylketoxime) silane
The invention relates to the field of chemical engineering, in particular to a refining method of methyltris (methylethylketoxime) silane. Modified activated carbon is used for adsorbing and refining a methyltris (methylethylketoxime) silane crude product, then a cation modified polyethersulfone filter membrane is used for finely filtering the product, the surface of the cation modified polyethersulfone filter membrane contains quaternary ammonium bond groups, and the cation modified polyethersulfone filter membrane has a good adsorption effect on hydroxyl compounds; a hydrolysis product methyltrihydroxysilane of the product can be adsorbed and removed to obtain a high-purity product; and the refined methyltris (methylethylketoxime) silane product obtained by the method disclosed by the invention is high in purity and light in chroma, and the market competitiveness of the product is greatly enhanced.
Owner:ZHEJIANG JINHUA NEW MATERIALS
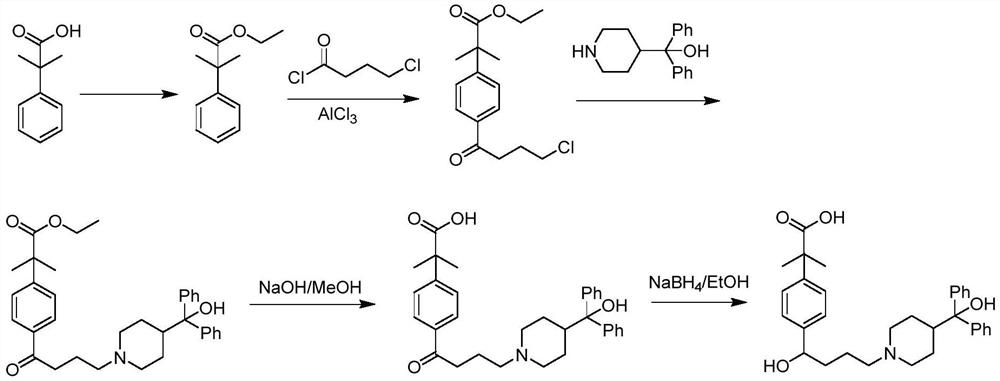
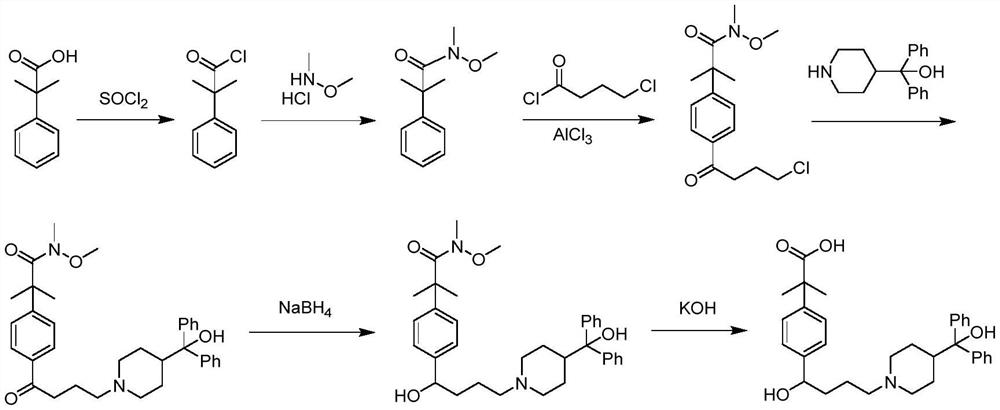
Preparation method of fexofenadine
PendingCN112661693AFew reaction stepsHigh yieldOrganic chemistryBulk chemical productionPropanoic acidChlorobenzene
The invention provides a preparation method of fexofenadine, which comprises the following steps: by using bromobenzene as a raw material, carrying out Friedel-Crafts acylation reaction to obtain 4'-bromo-4-chlorophenone ; enabling 4'-bromo-4-chlorobutanone and 1-methoxy-1-(trimethylsiloxy)-2-methyl-1-propene to subjected to coupling reaction to obtain 2-[4-(4 -chloro-1-butyryl)phenyl]-2-methyl methyl propionate; and sequentially carrying out N-alkylation, carbonyl reduction and alkaline hydrolysis on 2-[4-(4 -chloro-1-butyryl)phenyl]-2-methylpropanoate to obtain fexofenadine. The method has the advantages of cheap and easily available raw materials, easiness in operation, high yield, low cost, no meta-isomer, suitability for industrial production and the like.
Owner:NORTHWEST A & F UNIV
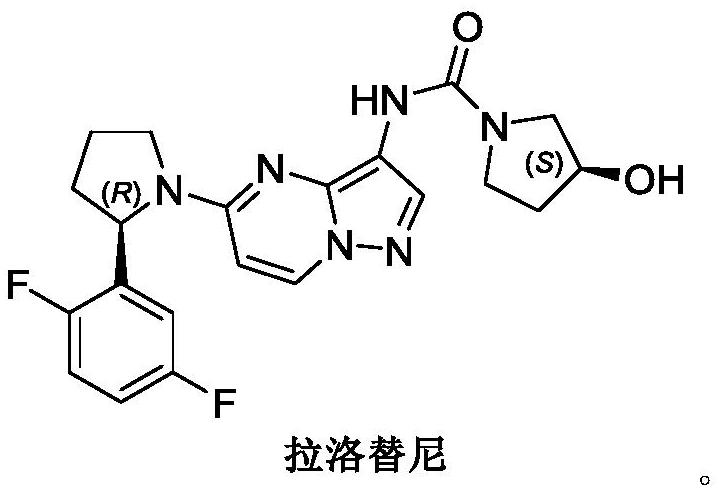
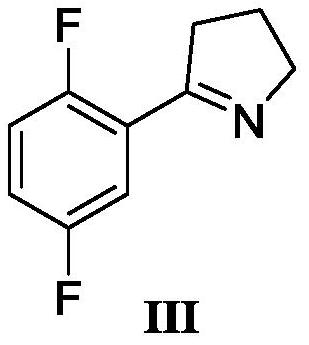
A kind of synthetic method of larottinib intermediate
ActiveCN111393347BRaw materials are easy to getEasy to operateOrganic chemistryDrugs synthesisPharmaceutical Substances
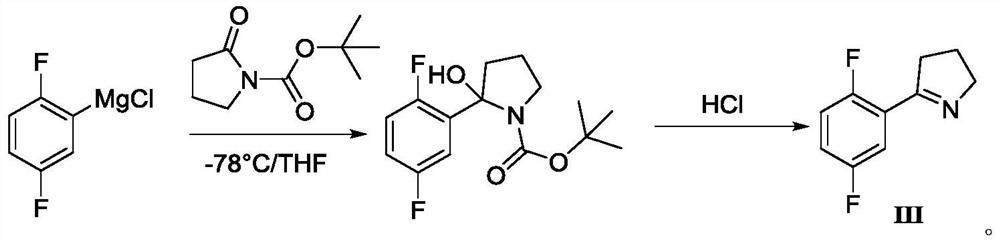
The invention discloses a synthesis method of a larotrectinib intermediate, and belongs to the field of drug synthesis. The method uses 4-chloro-1-(2, 5-difluorophenyl)butane-1-one (I) as the raw material to synthesize 5-(2, 5-difluorophenyl)-3, 4-dihydro-2H-pyrrole (III) through ammonolysis and ring closing one-pot method. The invention provides the synthesis method of the larotrectinib intermediate. The method is controllable in reaction process, easy to operate and high in yield.
Owner:ANHUI DEXINJIA BIOPHARM
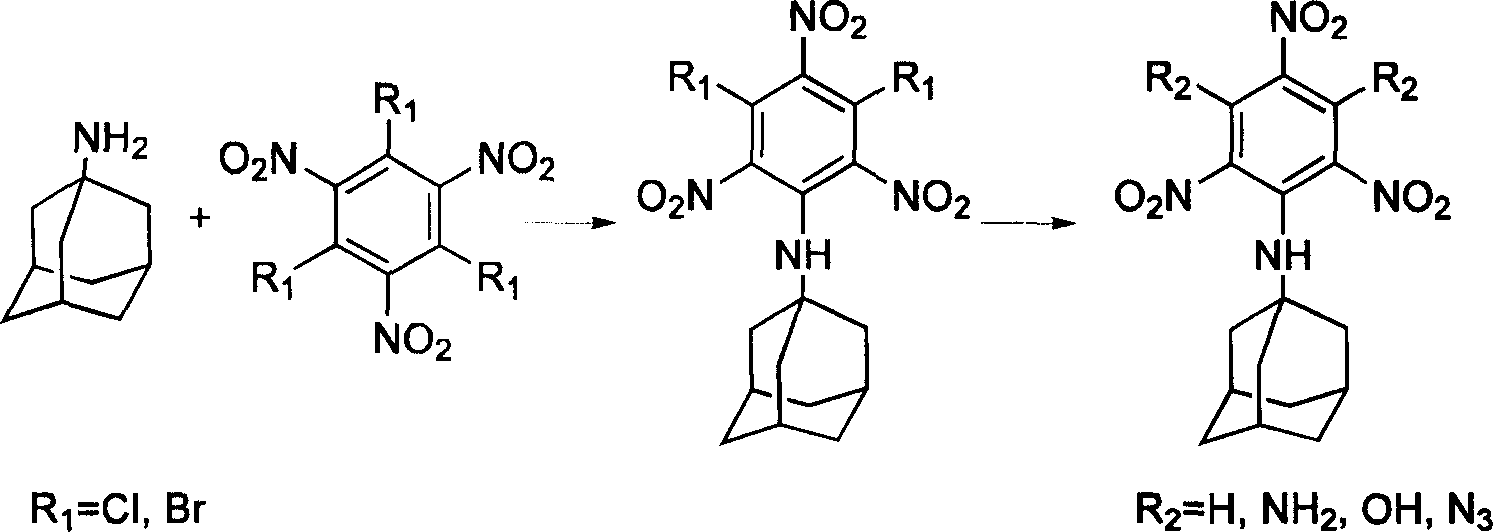
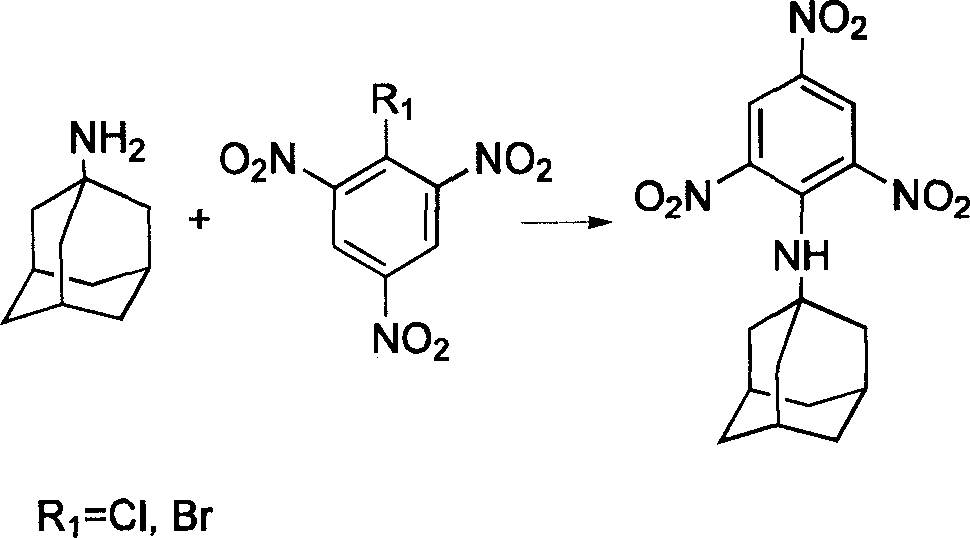
Compound containing multinitrobenzene amantadine and its synthesis method
InactiveCN1903836AIncrease the enthalpy of formationIncrease oxygen balance factorAmino preparation from aminesOrganic compound preparationSynthesis methodsSingle crystal
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com