Preparation method of methoxylamine hydrochloride
A technology of methoxyamine hydrochloride and hydrochloric acid, applied in oxime preparation, organic chemistry, etc., can solve problems such as safety, environmental protection, high toxicity, serious pollution, etc., to avoid low product yield and reduce toxic gas emissions , the effect of improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
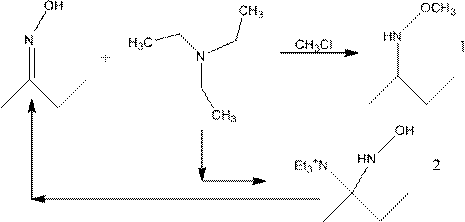
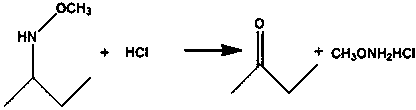
[0028] Add butanone oxime (C4H9NO) 43.5g (0.5mol), triethylamine (C6H15N) 70.8g (0.7mol), dimethyl sulfoxide (DMSO, C2H6OS) 87g (1.1mol) to 500ml reaction flask and heat up to 60 Slowly add 82g (0.65mol) of dimethyl sulfate (C2H6O4S) dropwise at ℃ for 50min, keep warm for 6h after the dropwise addition, transfer the reaction solution into a 500ml reaction bottle, add 78.2g (0.75mol) of 35% hydrochloric acid 100 Reaction at ℃ for 120min, cooling down to room temperature, crystallization and filtration to obtain the target product with a yield of 87%.
Embodiment 2
[0030] Add 52.3g (0.6mol) of butanone oxime (C4H9NO), 98g (0.97mol) of triethylamine (C6H15N), 111g (1.42mol) of dimethyl sulfoxide (DMSO, C2H6OS) to a 500ml reaction flask and heat up to 40°C Slowly add 77.3g (0.61mol) of dimethyl sulfate (C2H6O4S) dropwise for 90min, keep warm for 7h after the dropwise addition, transfer the reaction solution into a 500ml reaction bottle, add 93.7g (0.9mol) of 35% hydrochloric acid 120 The reaction was carried out at ℃ for 140 min, then cooled down to room temperature and crystallized and filtered to obtain the target product with a yield of 91%.
Embodiment 3
[0032] Add 61g (0.7mol) of butanone oxime (C4H9NO), 106.2g (1.05mol) of triethylamine (C6H15N), 122g (1.56mol) of dimethyl sulfoxide (DMSO, C2H6OS) to a 500ml reaction flask and heat up to 75°C Slowly add 123.6g (0.98mol) of dimethyl sulfate (C2H6O4S) dropwise for 30min, keep warm for 8h after the dropwise addition, transfer the reaction solution into a 500ml reaction bottle, add 104.3g (1mol) of 35% hydrochloric acid at 95°C After reacting for 110 minutes, the product was crystallized and filtered at room temperature to obtain the target product with a yield of 89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com