Method for synthesizing chiral nicotine from butyrolactone
A technology of nicotine and butyrolactone, applied in the direction of organic chemistry, can solve the problems of long reaction path, high production cost and low yield, and achieve the effects of shortening the reaction steps, mild reaction conditions and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
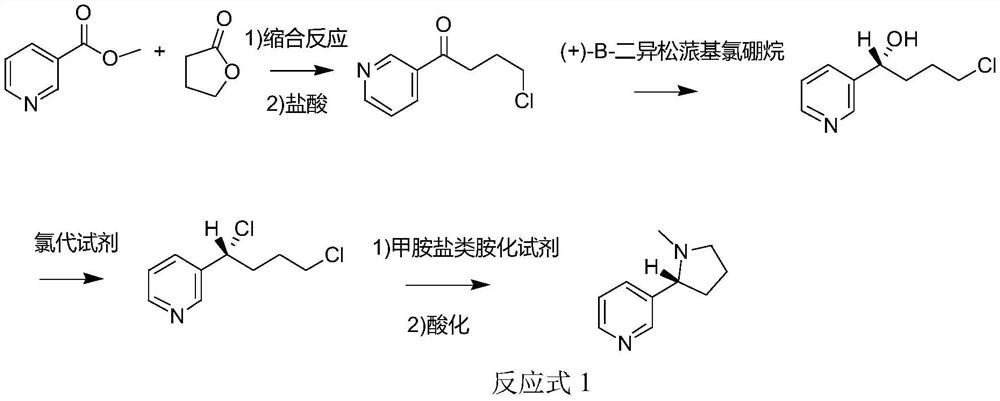
[0063] The method for synthesizing chiral nicotine from butyrolactone provided in Example 1, wherein the aminating agent is a methylamine salt-type aminating agent (specifically methylamine hydrochloride), and the synthetic route is shown in Reaction Formula 1, (S) -The specific preparation steps of nicotine are:
[0064] S1, at 0°C, add 86.1g (1mol, 1eq) γ-butyrolactone to 1L DMF, stir at 0°C for 10min, add 48g (2mol, 2eq) NaH, react at 0°C for 0.5h, then add 137.1g (1mol) methyl nicotinate was subjected to condensation reaction at 25°C for 2h, and the reaction was monitored by TCL until the end of the reaction to obtain a condensation product; 0.83L 12mol / L (1mol, 1eq) hydrochloric acid was added to the condensation product, and the reaction was refluxed at 80°C for 1h, Then add saturated brine for extraction, add sodium bicarbonate to make the pH of the system 7, extract 3 times with dichloromethane, combine the organic phases, spin dry to remove the solvent to obtain 4-chl...
Embodiment 2-3
[0068] Example 2-3 differs from Example 1 only in that: in the reaction of step S4, the types of methylamine salt amination reagents are adjusted, as shown in Table 1.
[0069] Table 1 Influence of methylamine salt amination reagent selection on (S)-nicotine yield
[0070] Numbering Methylamine salt amination reagent selection (S)-nicotine yield (%) Example 1 Methylamine hydrochloride 75 Example 2 Methylamine Sulfate 72 Example 3 Methylamine nitrate 70
Embodiment 4-7
[0071] Examples 4-7 differ from Example 1 only in that: in the reaction of step S4, the consumption of methylamine hydrochloride is adjusted, as shown in Table 2.
[0072] The influence of table 2 methylamine hydrochloride dosage selection on (S)-nicotine yield
[0073] Numbering Equivalents of methylamine hydrochloride (eq) (S)-nicotine yield (%) Example 1 3 75 Example 4 1 52 Example 5 2 68 Example 6 4 71 Example 7 5 70
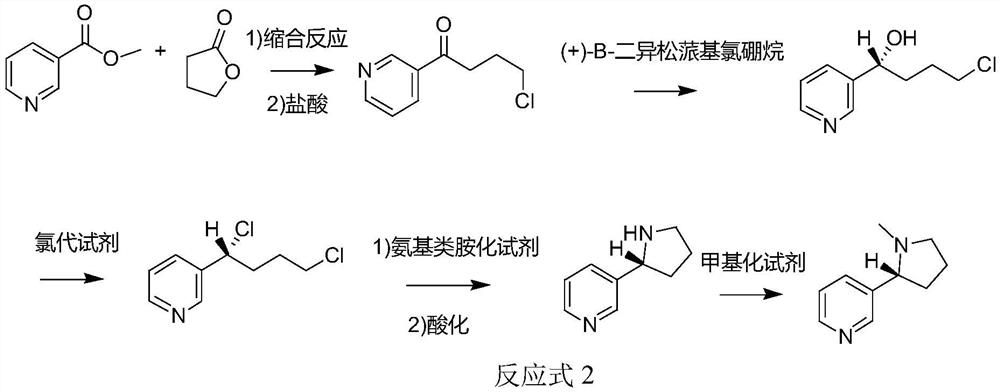
[0074] When the aminating reagent is an amino-based aminating reagent, the synthetic route of the method for synthesizing chiral nicotine from butyrolactone provided by the application is shown in Reaction Formula 2:
[0075]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com