Compound containing multinitrobenzene amantadine and its synthesis method
A technology of nitrobenzamantadine and its synthesis method, which is applied to the preparation of amino hydroxyl compounds, the preparation of amino compounds from amines, chemical instruments and methods, etc., which can solve the problems of increasing the oxygen balance coefficient and the difficulty of direct nitration of amantadine, etc. Achieve the effects of increasing oxygen balance coefficient, easy separation and purification, and short time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
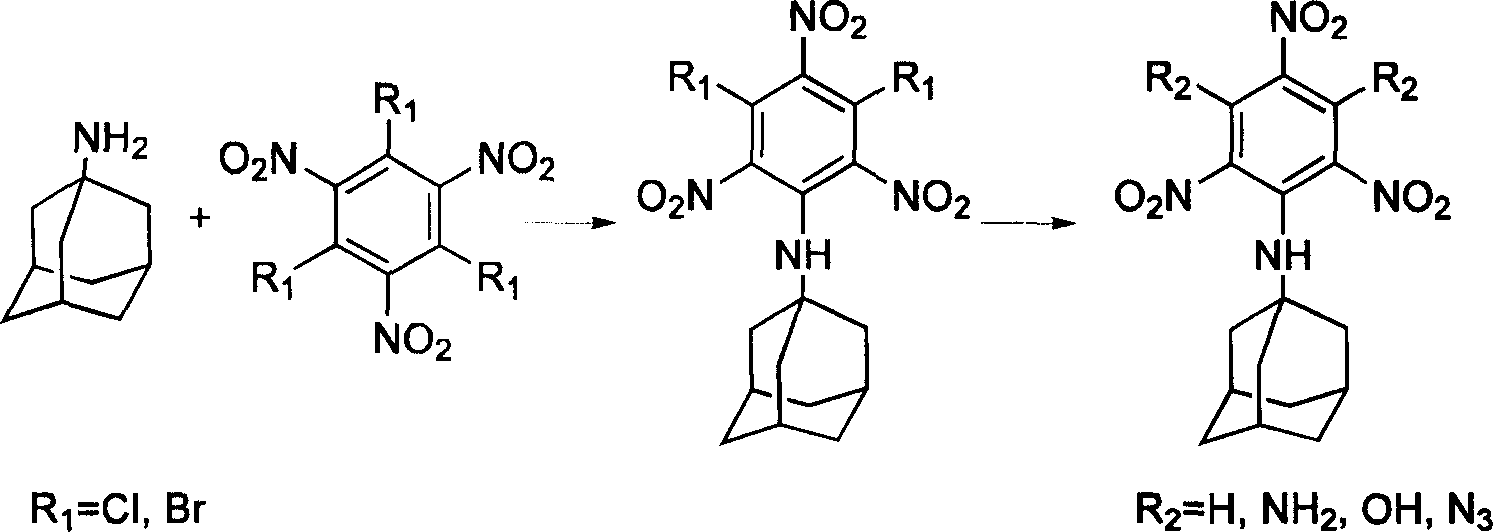
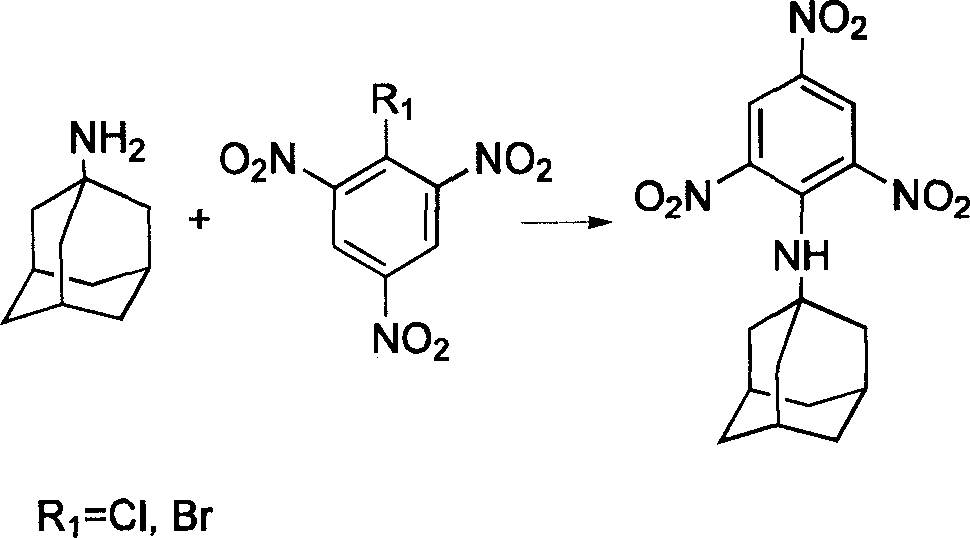
[0036] Synthetic method of diazidetrinitrophenylamantadine, the synthetic route is referring to accompanying drawing 1, the first step synthesizes dichlorotrinitrophenyladamantane, solvent-free solid-phase method, the trichlorotrinitrobenzene of 0.1mmol, 0.12mmol of amantadine was mixed and stirred, the mixture turned yellow, ultrasonically oscillated for 1 hour, poured into 10ml of cold water, and then added 5ml of aqueous hydrochloric acid solution with a concentration of 1mol / l to remove excess amantadine, and a uniform light yellow precipitate was obtained. Coagulate, filter, wash thoroughly, remove DMF, and dry in a water-bath oven at 40°C for more than 4 hours. Yield 88%.
[0037] In the second step, add 0.1mmol of dichlorotrinitrobenzamantadine into a 100ml flask with 30ml of acetonitrile, add 0.5mmol of sodium azide methanol solution into the equal pressure funnel, and react under reflux for 48 hours to determine the end point of the reaction , traceable with TLC. Co...
Embodiment 2
[0039] Synthetic method of trinitrophenylamantadine diazide, see accompanying drawing 1 for the synthetic route, the first step synthesizes dichlorotrinitrophenyladamantane, liquid phase reaction with solvent, 0.1mmol of trichlorotrinitrobenzene , 0.12mmol of amantadine was mixed in a 100ml flask containing 20ml of DMF, heated to 80°C for 8 hours, the mixture turned yellow, cooled to below 40°C, poured into 10ml of cold water, and then added 5ml of hydrochloric acid with a concentration of 1mol / l Aqueous solution, excess amantadine was removed to obtain a uniform light yellow precipitate, which was coagulated, filtered, fully washed, DMF removed, and dried in a water-bath oven at 40°C for more than 4 hours, with a yield of 88%.
[0040] For the second step, refer to the second step in Embodiment 1.
Embodiment 3
[0042] For the synthesis method of diaminotrinitrophenylamantadine, refer to accompanying drawing 1 for the synthetic route, and refer to the first step of Example 1 and the first step of Example 2 for the synthesis of dichlorotrinitrophenylamantadine. The second step is to add .1mmol of trichlorotrinitrobenzamantadine into a 100ml flask with 30ml of acetonitrile, add 1ml of ammonia water, and react under reflux for 26 hours. The judgment of the reaction end point can be tracked by TLC. Cold cut to below 40°C. Extract with 3×20ml of ethyl acetate, wash the organic phase with saturated ammonium chloride, and dry over anhydrous magnesium sulfate. Filtration and concentration gave a yellow solid. It was dried in a water-bath oven at 40° C. for more than 4 hours, weighed, and the yield was 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com