Compound containing tetrazine polynitrobenzene and synthesis method thereof
A technology of tetrazine trinitrobenzene and a synthesis method, which is applied to the compound containing tetrazine trinitrobenzene and the synthesis field thereof, can solve the problems of unstable energy-containing groups, difficulty in large-scale synthesis, and high production costs, and achieves the Easy to separate and purify, easy to synthesize in large quantities, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
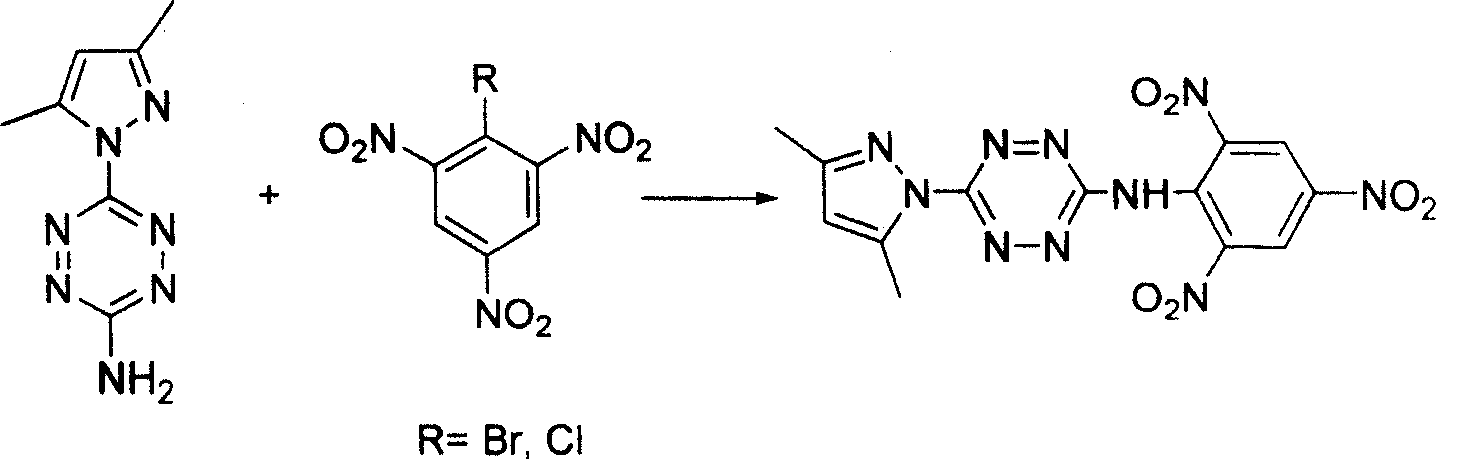
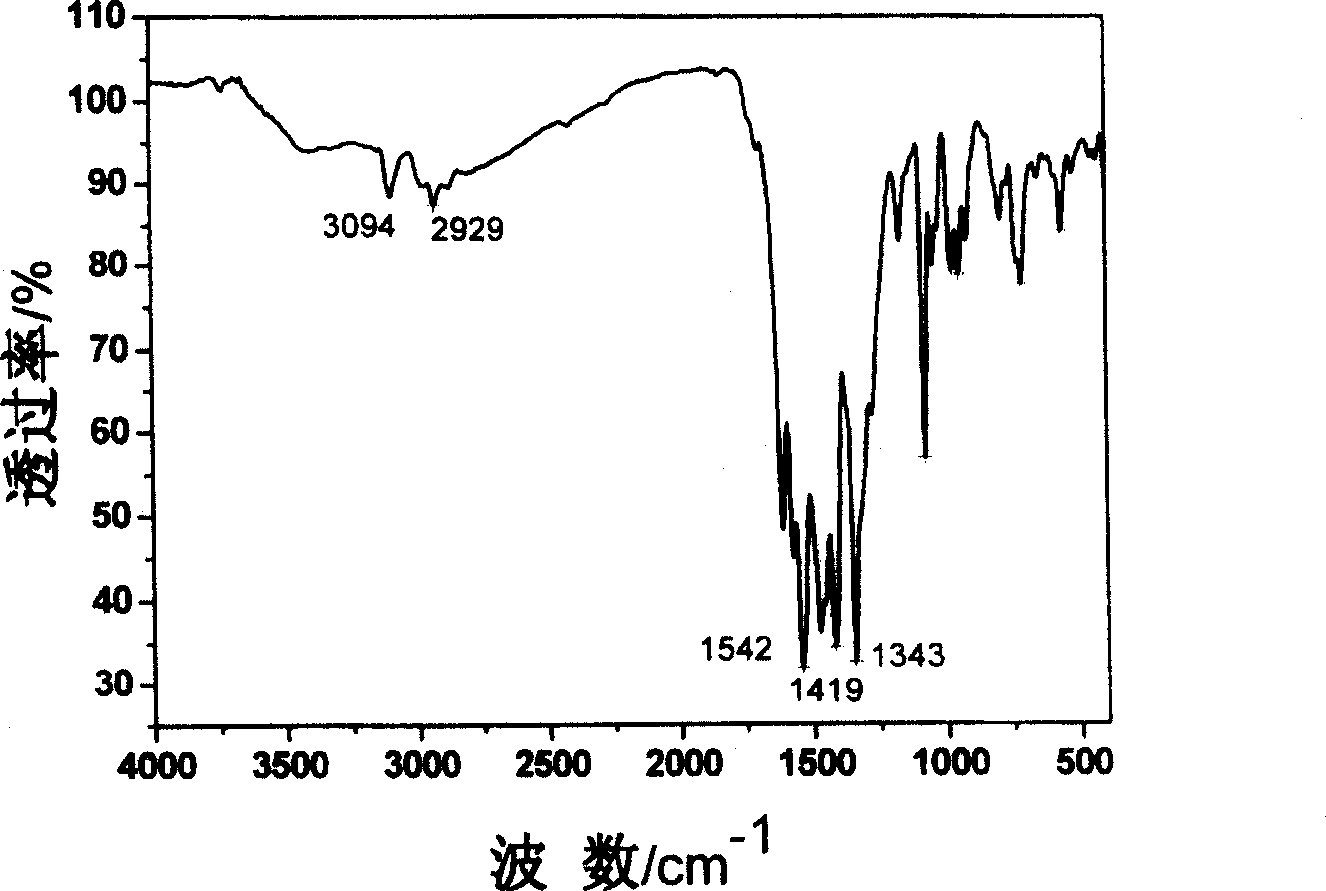
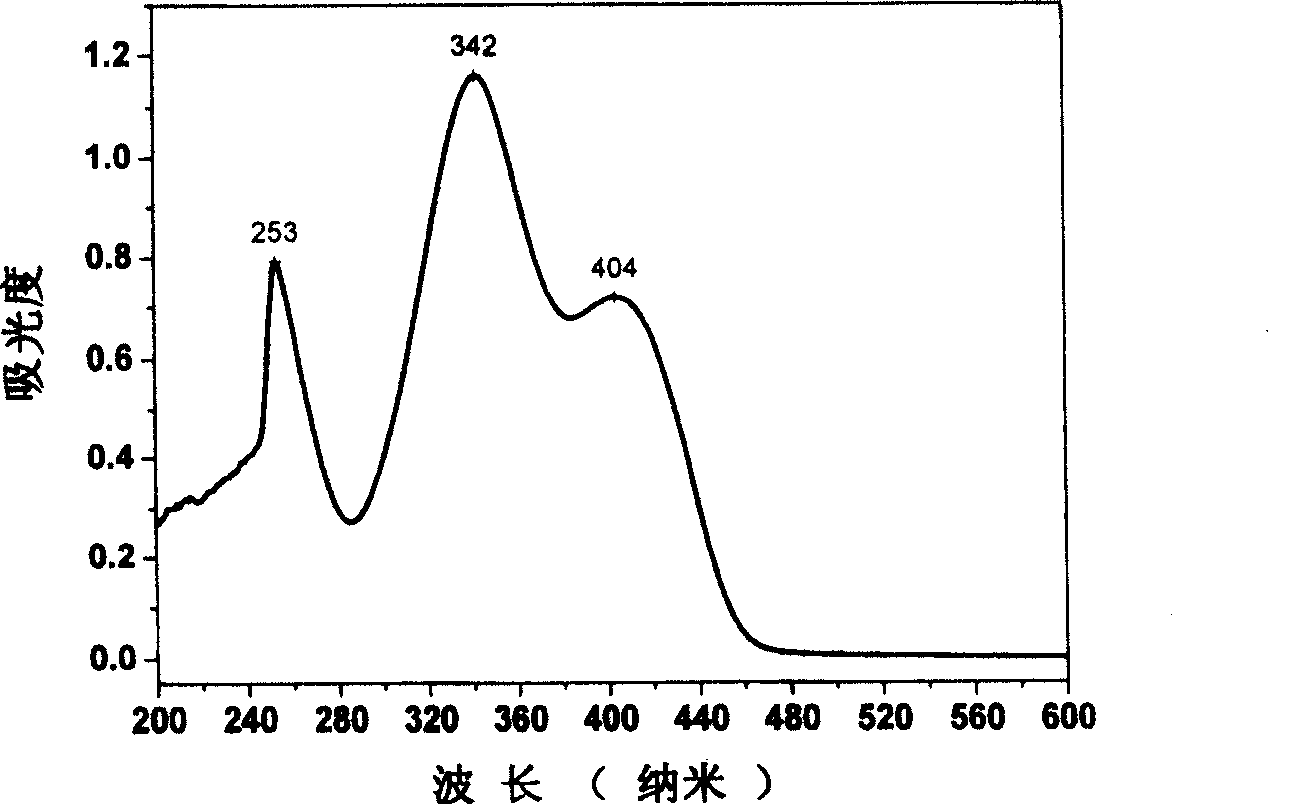
[0030] 3-imino-(2,4,6-trinitrobenzene)-6(3,5-dimethylpyrazole)-1,2,4,5-tetrazine synthesis method, the synthesis route is shown in Figure 1 , Dissolve 1.5mmol chlorotrinitrobenzene and 1mmol 3-amino-6(3,5-dimethylpyrazole)-1,2,4,5-tetrazine in 20mL of acetonitrile, heat at 80°C Reflux for 6 hours, stop the reaction and cool down to 20°C, concentrate by rotary evaporation, and purify by column chromatography to obtain a red precipitate. Dry in a water bath oven at 40 °C for over 1 hour. The product was dissolved in a mixed solvent of ethyl acetate and acetone with a molar ratio of 2, and slowly volatilized to obtain a red single crystal with a yield of 75% (based on chlorotrinitrobenzene). For the characterization, please see the accompanying drawing 2 for the infrared spectrum, the accompanying drawing 3 for the ultraviolet-visible spectrum, and the accompanying drawing 4 for the single crystal structure spectrum.
Embodiment 2
[0032] 3-imino-(2,4,6-trinitrobenzene)-6(3,5-dimethylpyrazole)-1,2,4,5-tetrazine synthesis method, the synthesis route is shown in Figure 1 , Dissolve 1.5mmol bromotrinitrobenzene and 1mmol 3-amino-6(3,5-dimethylpyrazole)-1,2,4,5-tetrazine in 20mL of acetonitrile, heat at 80°C Reflux for 2 hours, stop the reaction and lower the temperature to 20°C, concentrate by rotary evaporation, and purify by column chromatography to obtain a red precipitate. Dry in a water bath oven at 40 °C for over 1 hour. The product was dissolved in a mixed solvent of ethyl acetate and acetone with a molar ratio of 2, and slowly volatilized to obtain a red single crystal with a yield of 85% (based on bromotrinitrobenzene). For the characterization, please see the accompanying drawing 2 for the infrared spectrum, the accompanying drawing 3 for the ultraviolet-visible spectrum, and the accompanying drawing 4 for the single crystal structure spectrum.
Embodiment 3
[0034] 3-imino-(2,4,6-trinitrobenzene)-6(3,5-dimethylpyrazole)-1,2,4,5-tetrazine synthesis method, the synthesis route is shown in Figure 1 , 1.5mmol chlorotrinitrobenzene and 1mmol 3-amino-6(3,5-dimethylpyrazole)-1,2,4,5-tetrazine were dissolved in 20mL of N,N-dimethyl Dimethyl formamide, heated to reflux at 80°C for 4h, cooled to 20°C after stopping the reaction, extracted with ethyl acetate (3×20mL) and water, concentrated by rotary evaporation, and purified by column chromatography to obtain a red precipitate. Dry in a water bath oven at 40 °C for over 1 hour. The product was dissolved in a mixed solvent of ethyl acetate and acetone with a molar ratio of 2, and slowly volatilized to obtain a red single crystal with a yield of 78% (based on chlorotrinitrobenzene). For the characterization, please see the accompanying drawing 2 for the infrared spectrum, the accompanying drawing 3 for the ultraviolet-visible spectrum, and the accompanying drawing 4 for the single crystal st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com