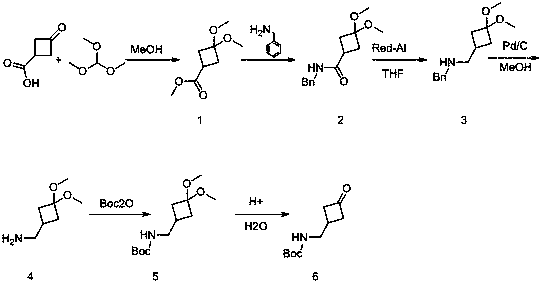
Method for synthesizing 3-(Boc-aminomethyl)cyclobutanone
A technology of aminomethylcyclobutanone and 3-boc-, which is applied in the field of synthesis of 3-Boc-aminomethylcyclobutanone, can solve the problems of troublesome post-processing and high cost of raw materials, and achieve simple post-processing and low reagent cost Low, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] step 1:
[0012] Add 3-oxocyclobutane carboxylic acid (45.0 g, 395 mmol) and methanol (250 mL) into a 500 ml three-necked flask; add trimethyl orthoformate (75 ml, 713 mmol) at room temperature; p-toluenesulfonic acid Monohydrate (2.0 g, 10.5 mmol). After heating and reflux stirring for 5 hours after the addition, the reaction solution was cooled to room temperature, most of the methanol was spun off, a saturated sodium bicarbonate solution (500 mL) was added, ethyl acetate (300 mL*2) was added for extraction, the organic phase was separated, and water (300 mL) and saturated brine (300 ml), and dried over anhydrous sodium sulfate. The filtrate was spin-dried to obtain a colorless oily liquid, the target compound 1 (71.5 g, 411 mmol, 104%). 1 H NMR (400 MHz, CDCl 3 ):3.71 (s, 3H), 3.17 (d, J=8.2 Hz, 6H), 2.90(p, J=8.7 Hz, 1H), 2.49-2. 36 (m, 4H).
[0013] Step 2:
[0014] Add compound 1 (60.0 g, 344 mmol) and methanol (500 mL) into a 1000 ml three-neck flask; add be...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com