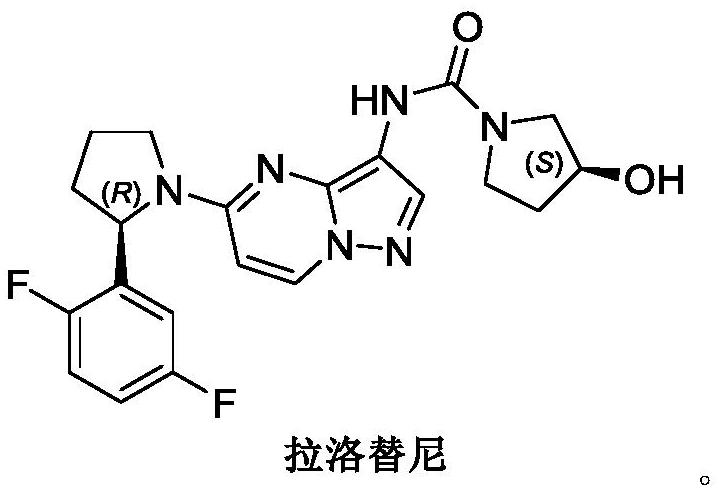
A kind of synthetic method of larottinib intermediate
A synthetic method and intermediate technology, applied in the field of drug synthesis, can solve the problems of difficult product purification and difficult removal of triphenylphosphine oxide, and achieve the effects of reduced production costs, easy availability of raw materials, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
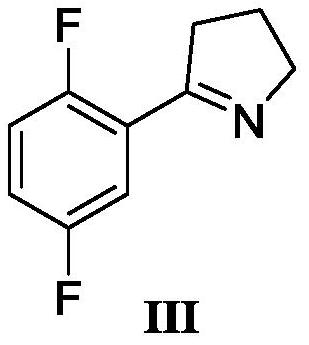
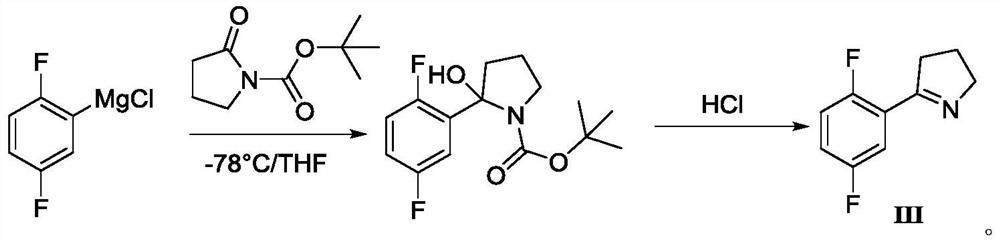
[0026] Add 50g of 4-chloro-1-(2,5-difluorophenyl)butan-1-one (1eq) (purchased from Shanghai Haohong Biomedical Technology Co., Ltd.) into a 500mL autoclave, add 200mL of tetrahydrofuran, and pass Add liquid ammonia, maintain the pressure of the reaction system at 0.4 MPa, react at 55°C for 4 hours, cool to room temperature 20-25°C, add 25mL of water under normal pressure, then slowly add formic acid dropwise, adjust the pH to 4.0, and continue stirring at room temperature for 4-5 hours , concentrate the solvent, add water 100mL and 150mL ethyl acetate to extract, separate layers, wash the organic phase with water once, dry, and concentrate to obtain a colorless or light yellow oil, namely 5-(2,5-difluorophenyl)-3, 4-Dihydro-2H-pyrrole (III), the two-step total yield is 93%, and the gas phase purity is 98.5%.
Embodiment 2
[0028] Add 50g of 4-chloro-1-(2,5-difluorophenyl)butan-1-one (1eq) (purchased from Shanghai Haohong Biomedical Technology Co., Ltd.) into a 500mL autoclave, add 250mL of ethanol, and pass Add liquid ammonia, maintain the pressure of the reaction system at 0.5 MPa, react at 60°C for 4 hours, cool to room temperature 20-25°C, add 25mL of water under normal pressure, then slowly add formic acid dropwise, adjust the pH to 3.5, and continue stirring at room temperature for 4-5 hours , concentrate the solvent, add water 100mL and 150mL ethyl acetate to extract, separate layers, wash the organic phase with water once, dry, and concentrate to obtain a colorless or light yellow oil, namely 5-(2,5-difluorophenyl)-3, 4-Dihydro-2H-pyrrole (III), the two-step total yield is 92%, and the gas phase purity is 98.2%.
Embodiment 3
[0030] Add 50g of 4-chloro-1-(2,5-difluorophenyl)butan-1-one (1eq) (purchased from Shanghai Haohong Biomedical Technology Co., Ltd.) into a 500mL autoclave, add 250mL of ethanol, and pass Add liquid ammonia, normal pressure, react at 60°C for 4 hours, and the liquid phase control shows no reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com