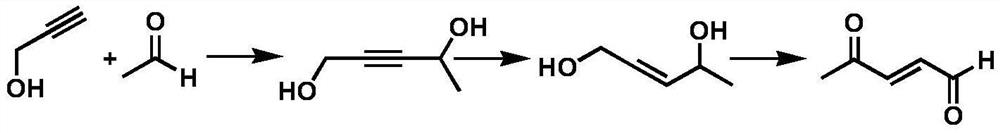
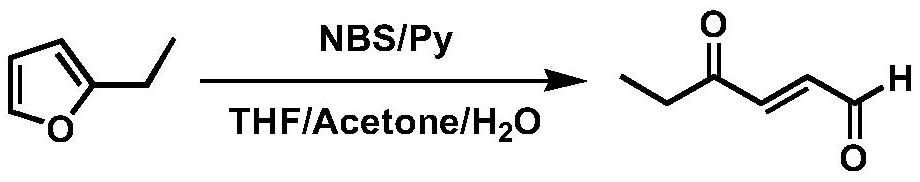
Synthesis method of trans-4-oxo-2-hexenal
A synthesis method and hexenal technology, applied in the field of chemical production, can solve problems such as the bottleneck of the synthesis method, and achieve the effects of less equipment investment, uniform temperature and controllable reaction conditions, and high safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example Construction
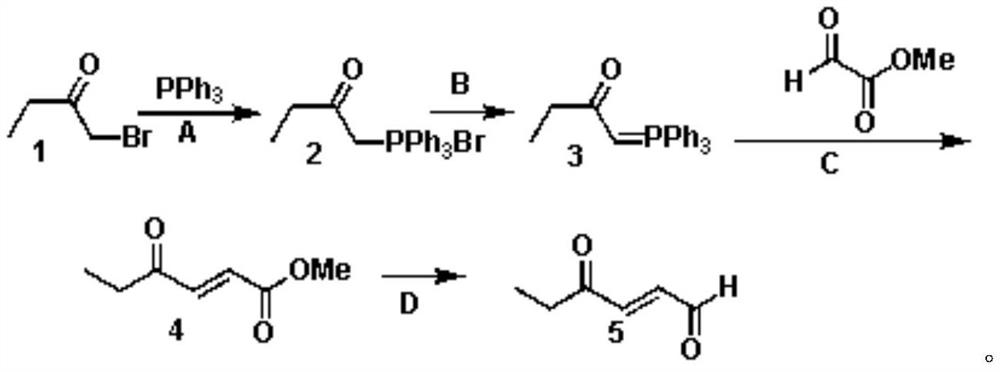
[0030] Specifically, its synthetic method includes:
[0031] 1. Synthesis Step A: Synthesis of 2-oxobutyltriphenylphosphonium bromide salt
[0032] Make bromobutanone (compound 1) and triphenylphosphine with a molar ratio of 1:1.2~1.5 in an organic solvent (dichloromethane, tetrahydrofuran, methyl tert-butyl ether, benzene, methyl tetrahydrofuran, toluene, acetonitrile or At least one of diisopropyl ether) is heated to reflux reaction, cooled and suction filtered, and dried to obtain 2-oxobutyltriphenylphosphonium bromide salt (compound 2);
[0033] 2. Synthesis Step B: Synthesis of (Ethylcarbonylmethylene) Triphenylphosphine
[0034] The 2-oxobutyl triphenylphosphonium bromide salt (compound 2) is dissolved in an organic solvent (dichloromethane, tetrahydrofuran, methyl tert-butyl ether, benzene, methyl tetrahydrofuran, toluene, acetonitrile or dichloromethane At least one in isopropyl ether), according to the molar ratio of 2-oxobutyltriphenylphosphonium bromide salt and a...
Embodiment 1
[0041] Synthesis of 2-oxobutyltriphenylphosphonium bromide salt (compound 2):
[0042] Dissolve triphenylphosphine (31.5g, 0.12mol) in 150mL tetrahydrofuran, and add bromobutanone (15.1g, 0.1mol) / 50mL tetrahydrofuran solution dropwise under ice-water cooling; after the dropwise addition, stir for 1 hour and heat to reflux Cool to 0°C for 2 hours to crystallize, filter with suction, wash the filter cake with petroleum ether, and dry with an infrared lamp to obtain 39.3 g of 2-oxobutyltriphenylphosphonium bromide salt with a yield of 95%.
Embodiment 2
[0044] Synthesis of (ethylcarbonylmethylene)triphenylphosphine (compound 3):
[0045] Suspend 2-oxobutyltriphenylphosphonium bromide salt (82.6g, 0.2mol) in 500mL ethyl acetate, under vigorous stirring, add potassium tert-butoxide (24.6g, 0.22mol) in batches; Stir for 30 minutes, add ice water and stir rapidly for 5 minutes, statically separate layers, dry the organic phase with anhydrous sodium sulfate, and concentrate to obtain 61 g of (ethylcarbonylmethylene)triphenylphosphine with a yield of 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com