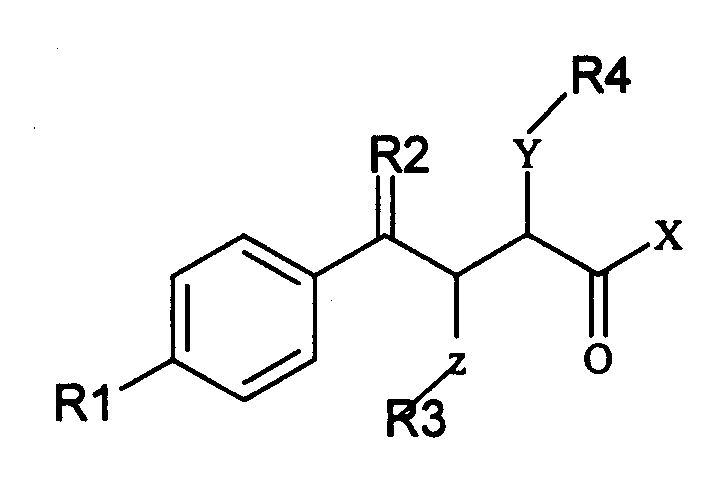
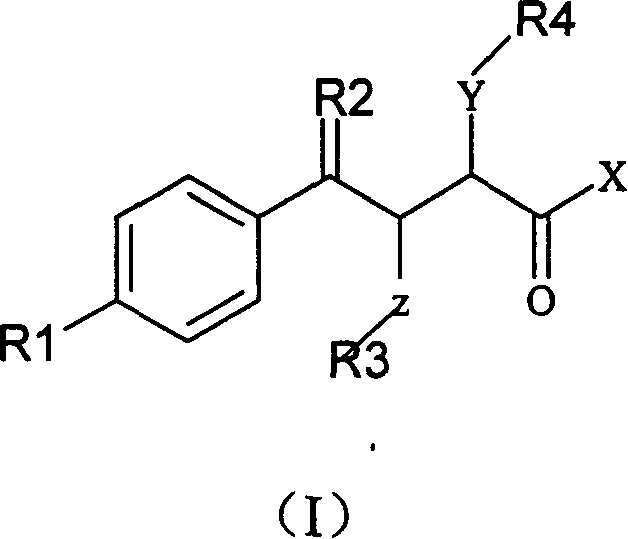
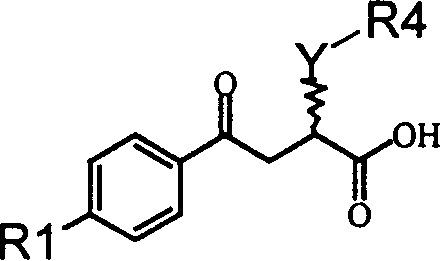
Alpha position heteroatom substituted gamma aryl ketobutyric acid derivative, process, pharmaceutical combination and uses thereof
A compound and heterocyclic group technology, applied in the field of butanone acid derivatives, can solve the problems of skeletal muscle and joint damage, no obvious anti-tumor or anti-inflammatory effect, no significant curative effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0171] 2-(2-Hydroxyethylamino)-4-oxo-4-(4'-biphenyl)-butyric acid (A): Preparation of 3-(4-phenylbenzoyl)acrylic acid:
[0172] Dissolve 23.13 grams of biphenyl and 14.71 grams of maleic anhydride in 300 milliliters of dichloromethane, add 40.00 grams of anhydrous aluminum trichloride in four batches under cooling in an ice-water bath, slowly rise to room temperature, continue to react for 5 hours, and concentrate To dryness, add a mixture of crushed ice and concentrated hydrochloric acid to the obtained viscous liquid, carry out hydrolysis, filter the obtained yellow solid, wash with water until neutral, then wash several times with petroleum ether, after draining, recrystallize with glacial acetic acid to obtain 37.26 gram of yellow crystals, yield: 93.19%, melting point: 168-170°C (literature value: 172-175°C).
[0173] (B): Preparation of 2-(2-hydroxyethylamino)-4-oxo-4-(4'-biphenyl)-butyric acid:
[0174] Suspend 5.045 grams of 3-(4-phenylbenzoyl)acrylic acid in 100 mill...
Embodiment 2
[0177] 2-Benzylamino-4-oxo-4-(4’-biphenyl)-butanoic acid
[0178] The method is the same as in Example 1 (B), except that 5.434 grams of 3-(4-phenylbenzoyl) acrylic acid, 2.308 grams of benzylamine, and 100 milliliters of ether are used as solvent to obtain 6.877 grams of white powdery solid, yield: 88.83%, melting point: 177-179°C (decomposition).
[0179] 1 HNMR (300MHz, DMSO): δ (ppm) = 3.361 (dd, 1H, J: 7.5Hz, 17.1Hz), 3.456 (dd, 1H, J: 5.4Hz, 17.1Hz), 3.654 (t, 1H, J: 5.4Hz, 6.9Hz), 3.87 (d, 1H, J: 13.5Hz), 3.968 (d, 1H, J: 13.2Hz), 7.159-8.055 (m, 14H).
Embodiment 3
[0181] 2-n-Butylamino-4-oxo-4-(4’-biphenyl)-butanoic acid
[0182] The method is the same as in Example 1 (B), except that 1.261 grams of 3-(4-phenylbenzoyl) acrylic acid is used, 0.366 grams of n-butylamine, and 25 milliliters of ether are used as solvents to obtain 1.502 grams of white powdery solids, yield : 92.32%, melting point: 172.6-174.0°C (decomposition).
[0183] 1 HNMR (300MHz, DMSO): δ (ppm) = 0.867 (t, 3H, J: 7.5Hz), 1.303 (q, 2H, J: 7.5Hz), 1.541 (m, 2H), 2.742 (t, 1H, J : 7.5Hz), 2.883(dd, 1H, J: 7.2Hz, 13.2Hz), 3.552(m, 2H), 3.679(m, 1H), 7.486(m, 3H, J: 7.5Hz), 7.745(d, 2H, J: 7.5), 7.836 (d, 2H, J: 8.4Hz), 8.039 (d, 2H, J: 8.4Hz).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com