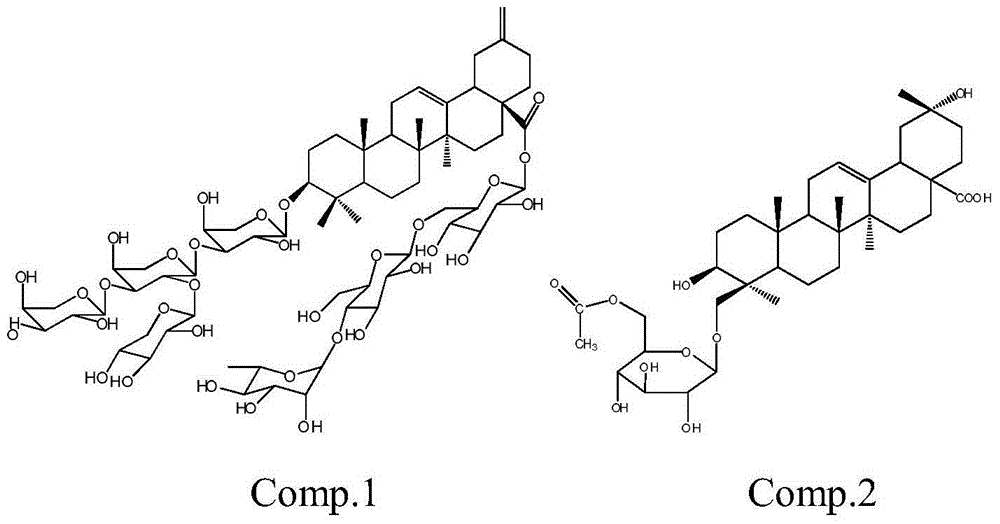
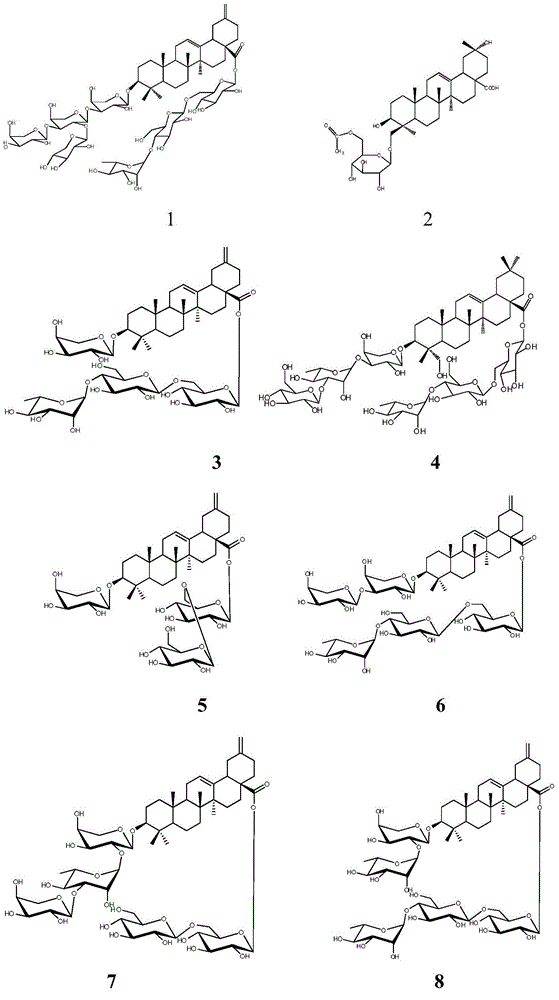
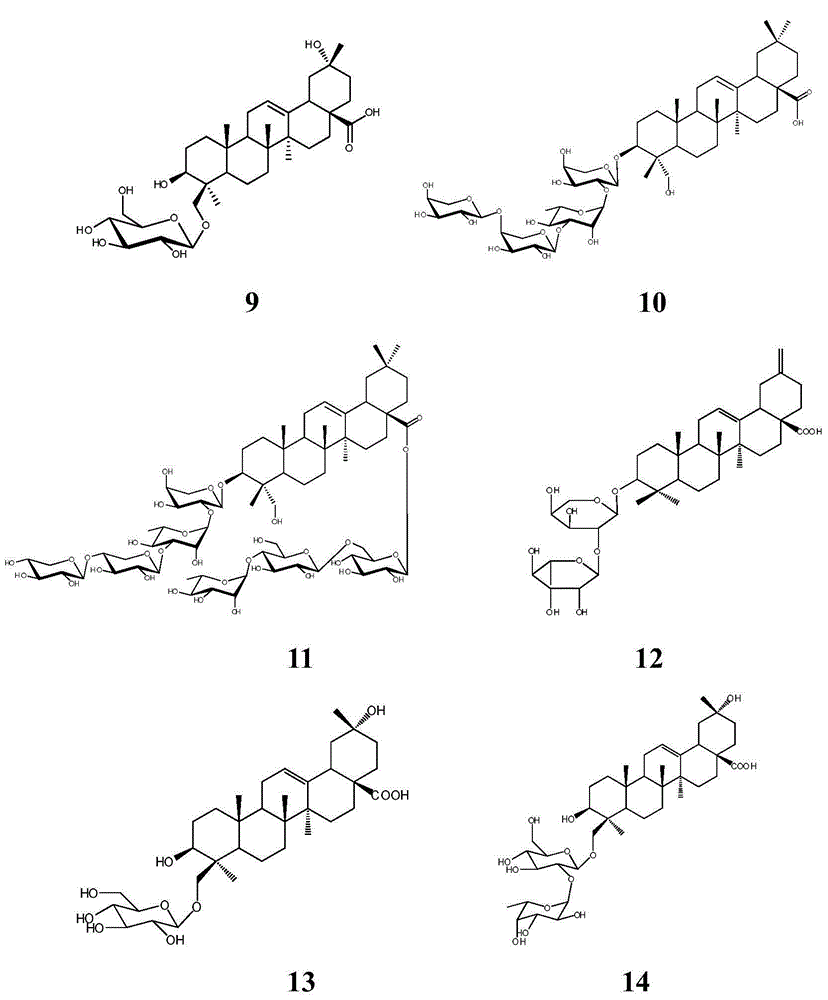
Methyl reduced oleanane triterpenoid saponin, preparation method of its active composition and application thereof
A technology for normethyloleanane and methyloleanane is applied in the field of preparation and application of normethyloleanane-type triterpene saponins and active compositions thereof, and can solve problems such as limited application and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Huanglaguo root 4kg, crushed and extracted three times with 12 times, 10 times and 8 times of 35% alcohol at 80°C, filtered (3 hours for the first time, 2 hours for the second time, 2 hours for the third time) and combined The filtrate was recovered from the solvent or evaporated to dryness until the alcohol concentration was less than 5%, diluted with water to 4 liters, and extracted with petroleum ether, dichloromethane, ethyl acetate, and n-butanol at a ratio of 1:2, and extracted 4 liters respectively. Once, the n-butanol extract was combined, the solvent was reclaimed, and the extract was obtained, which was separated by silica gel column chromatography, and 1.5 grams of silica gel was added for every gram of extract to mix the sample packing column, and the dichloromethane / methanol system 20:1, 15:1 , 12:1, 10:1, 7:1, 5:1, 3:1, 2:1 gradient elution. Fractions of 10:1-2:1 were separated by ODS, methanol / water 1:9, 2:8, 3:7, 5:5, 6:4, 7:3 gradient elution, compound ...
Embodiment 2
[0030] Huanglaguo root 4kg, crushed and extracted three times with 12 times, 10 times and 8 times of 95% alcohol at room temperature, (4 hours for the first time, 4 hours for the second time, 3 hours for the third time) filtered, combined Filtrate, recover the solvent or evaporate to dryness until concentrated to no alcohol smell, add water to dilute to 2 liters, use non-polar macroporous adsorption resin D-101 to separate, mobile phase is methanol / water, pass through water, 40%, 85% in turn Methanol was eluted to obtain 40% alcohol eluate, further separated by silica gel column chromatography, adding 1.5 grams of silica gel for every gram of extract and mixing sample packing, using dichloromethane / methanol system 20:1, 15:1, 12: 1, 10:1, 7:1, 5:1, 3:1, 2:1 gradient elution. Take the fraction of 10:1-2:1 and use ODS to separate it, and pass 1:15, 1:10, 3:20, 1:5, 1:4, 1:3, 2:5, 1:1, 4: 3 was eluted with an acetonitrile / water gradient, and compounds 2 (5.6 mg) and 1 (4.3 mg) w...
Embodiment 3
[0032] 3 kg of yellow wax fruit stems, heated and extracted three times at 80°C with 12 times, 10 times and 8 times of 60% alcohol after crushing, (4 hours for the first time, 3 hours for the second time, 3 hours for the third time) filtered, combined Filtrate, recover the solvent or evaporate to dryness until the alcohol concentration is less than 5%, add water to dilute to 6 liters, extract with cyclohexane, chloroform, ethyl acetate, n-butanol at a ratio of 1:1, and extract twice respectively, the target compound It is mainly enriched in the n-butanol layer, the n-butanol extract is combined, the solvent is recovered, and the extract is obtained, which is separated by silica gel column chromatography, and 1.5 grams of silica gel is added to each gram of the extract to mix the sample and packed into the column, and the dichloromethane / methanol system is used 20:1, 15:1, 12:1, 10:1, 7:1, 5:1, 3:1, 2:1 gradient elution. Take the fraction of 10:1-2:1 and use ODS to separate it, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com