Amine dehydrogenase mutant and application thereof in synthesis of chiral amine alcohol compound
A technology for alcohol compounds and chiral amines, which can be used in applications, microorganism-based methods, and introduction of foreign genetic material using carriers, which can solve the problems of harsh reaction conditions, low safety factor, and large pollution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
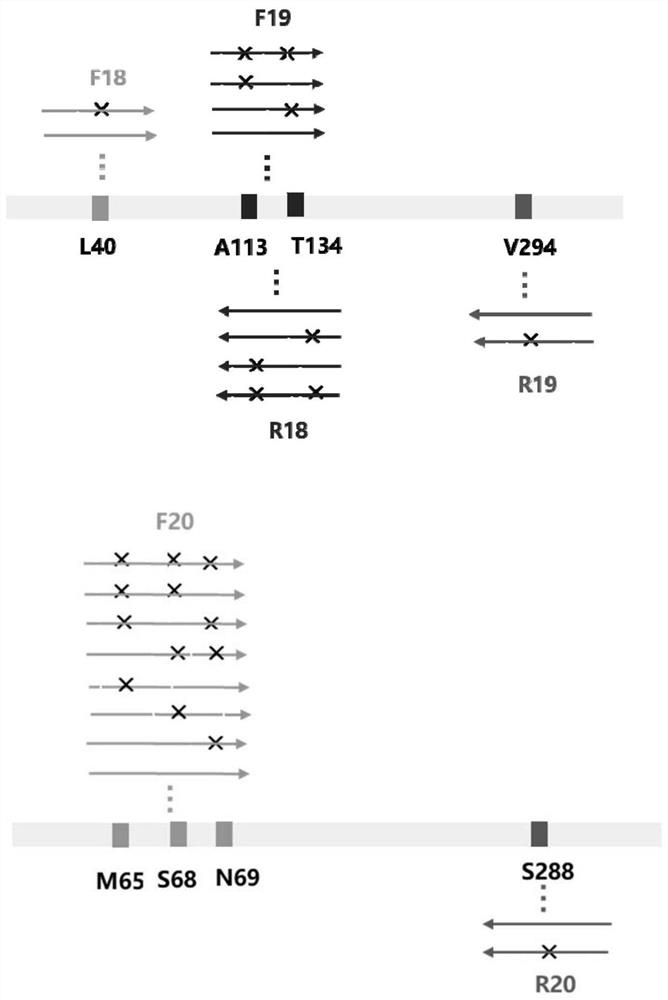
Image
Examples
Embodiment 1
[0202] Example 1. Preparation of engineering bacteria of amine dehydrogenase SpAmDH gene or its mutant
[0203] 1. Preparation of amine dehydrogenase SpAmDH genetically engineered strains
[0204] The coding gene of amine dehydrogenase (SpAmDH) derived from Sporarcina psychrophila was codon-optimized using Escherichia coli as the host cell to obtain the SpAmDH gene shown in SEQ ID No. 1 in the sequence listing. The amino acid sequence of SpAmDH is shown in SEQ ID No. 2 in the sequence listing.
[0205] The SpAmDH gene shown in SEQ ID No.1 in the full gene synthesis sequence table, the small DNA fragment between the recognition sequences of NdeI and XhoI in the pET24a(+) vector (Novagen) was replaced with the SpAmDH gene shown in SEQ ID No.1, A recombinant vector was obtained, which was designated as pET24a-SpAmDH. pET24a-SpAmDH could express SpAmDH shown in SEQ ID No. 2 in the sequence listing, and the expression of SpAmDH gene was driven by T7 promoter.
[0206] The recombi...
Embodiment 2
[0239] Example 2. Expression of amine dehydrogenase SpAmDH or its mutant and preparation of whole cells, crude enzyme powder and pure enzyme solution
[0240] The recombinant bacteria BL21(DE3) / pET24a-SpAmDH, BL21(DE3) / pET24a and each amine dehydrogenase SpAmDH gene mutant engineering strain prepared in Example 1 were induced to express, and the whole cells and crude enzyme powder of each strain were obtained. With pure enzyme solution, the operation steps for each strain are as follows:
[0241] Pick the strains into 5mL LB liquid medium containing 50μg / mL kanamycin, shake at 37°C and 220rpm overnight for 12h, and then inoculate TB containing 50μg / mL kanamycin according to the volume percentage of 1% inoculum. In liquid medium, cultivate to OD at 37°C 600 When it is 0.7, IPTG with a final concentration of 0.1 mmol / L was added. After inducing expression at 20°C and 220rpm for 12h, centrifuge at 4°C and 4,000rpm for 10min to collect the precipitated cells (that is, whole cells...
Embodiment 3
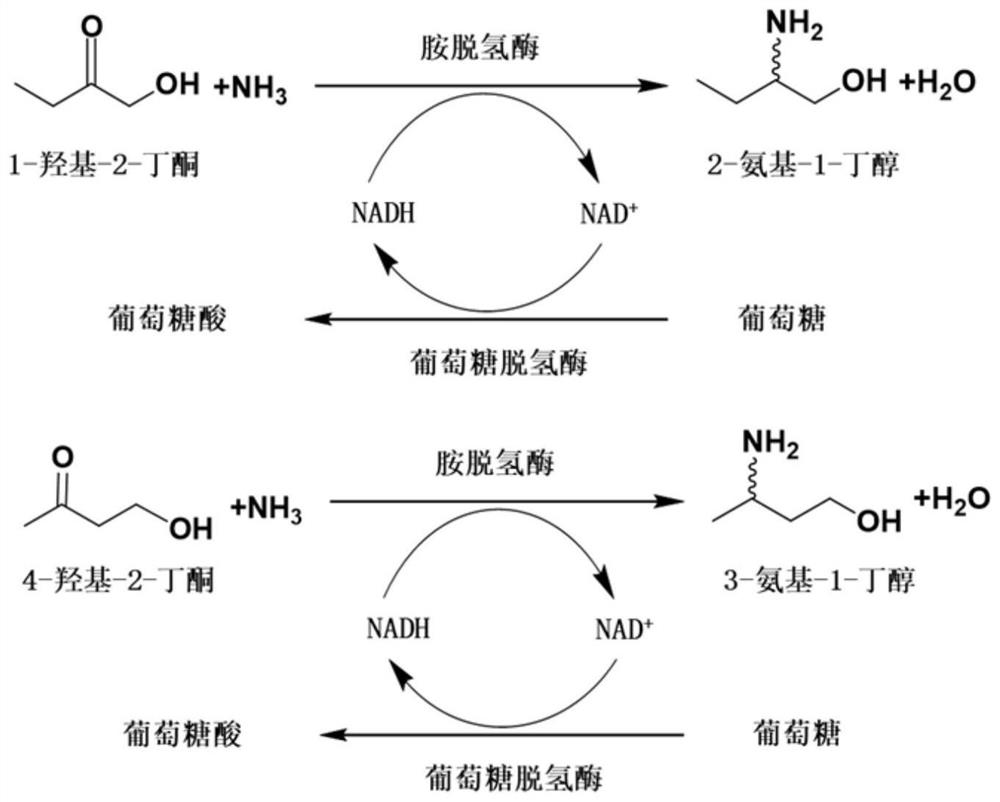
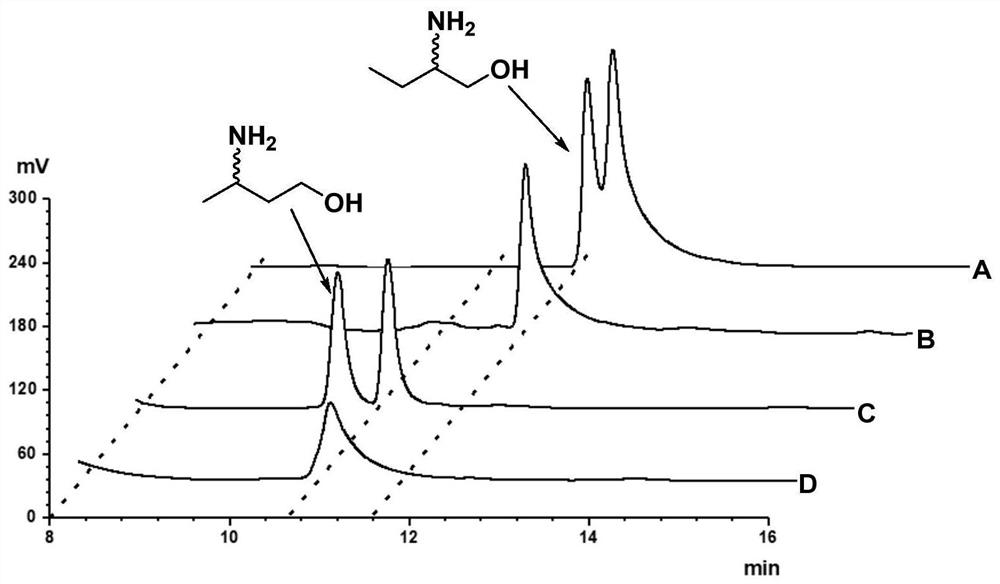
[0242] Example 3. Amine dehydrogenase SpAmDH or its mutant catalyzes the generation of (S)-2-amino-1-butanol from 1-hydroxy-2-butanone
[0243] The crude enzyme powder or whole cell of the amine dehydrogenase SpAmDH or its mutant prepared in Example 2 is used to catalyze 1-hydroxy-2-butanone to generate (S)-2-amino-1-butanol, as shown in the schematic diagram. figure 2 shown, and BL21(DE3) / pET24a was used as a control.
[0244] The reaction system of the catalytic reaction of SpAmDH or its mutant is obtained by adding the following amount of substances to 1 mol / L ammonium chloride / ammonia buffer solution (a mixture of ammonium chloride and ammonia in an equimolar ratio, pH 8.5): Substrate 1 -Hydroxy-2-butanone 20mmol / L or 40mmol / L, amine dehydrogenase SpAmDH or its mutant crude enzyme powder 20g / L or whole cell 100g / L, NAD + (Exist in the form of oxidized coenzyme I aqueous solution) 1mmol / L, GDH crude enzyme powder 2g / L, glucose 100mmol / L, lysozyme 1g / L (Beijing Soleibao Te...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific vitality | aaaaa | aaaaa |
| Specific vitality | aaaaa | aaaaa |
| Km value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com