Method for preparing cetrorelix
A technology for the preparation of cetrorelix, which is applied in the field of preparation of polypeptide drugs, can solve the problems of high cost, low synthetic purity, and unsuitability for industrial scale production, and achieve the effect of low cost and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Example 1: Preparation of Cetrorelix AM resin
[0084] Weigh 12.5g (10mol) of Rink Amide AM resin with a substitution degree of 0.80mmol / g, add it to a solid-phase reaction column, wash it once with DMF, and swell the AM resin with DCM for 30 minutes, then use DMF:pyridine with a volume ratio of 4 :1 mixed solution to remove Fmoc protection, then wash with DMF 6 times, weigh 9.34g Fmoc-D-Ala-OH (30mmol), 4.05g HOBt (30mmol) and add DCM and DMF with a volume ratio of 1:1 to mix Solution, add 3.79g DIC (30mmol) under ice-water bath to activate, then add to the above-mentioned reaction column equipped with resin, react at room temperature for 2 hours, use ninhydrin method to detect and judge the reaction end point, if the resin is colorless and transparent, it means The reaction is complete; if the resin develops color, it means that the reaction is incomplete and needs to be reacted for another 1 hour. This judgment standard is applicable to the detection and judgment of ...
Embodiment 2
[0087] Example 2: Large-scale preparation of Cetrorelix AM resin
[0088] Weigh 125.00g (100mol) of Rink Amide AM resin with a substitution degree of 0.80mmol / g, add it to a solid-phase reaction column, wash it once with DMF, and swell the AM resin with DCM for 30 minutes, then use DMF:pyridine with a volume ratio of 4 : 1 mixed solution to remove Fmoc protection, then wash 6 times with DMF, weigh 93.40g Fmoc-D-Ala-OH (300mmol), 40.52g HOBt (300mmol) and add DCM and DMF with a volume ratio of 1:1 to mix Solution, add 37.92g DIC (300mmol) under ice-water bath to activate, then add to the above-mentioned reaction column equipped with resin, react at room temperature for 2 hours, use ninhydrin method to detect and judge the reaction end point, if the resin is colorless and transparent, it means The reaction is complete; if the resin develops color, it means that the reaction is incomplete and needs to be reacted for another 1 hour. This judgment standard is applicable to the dete...
Embodiment 3
[0091] Example 3: Preparation of Cetrorelix Crude Peptide
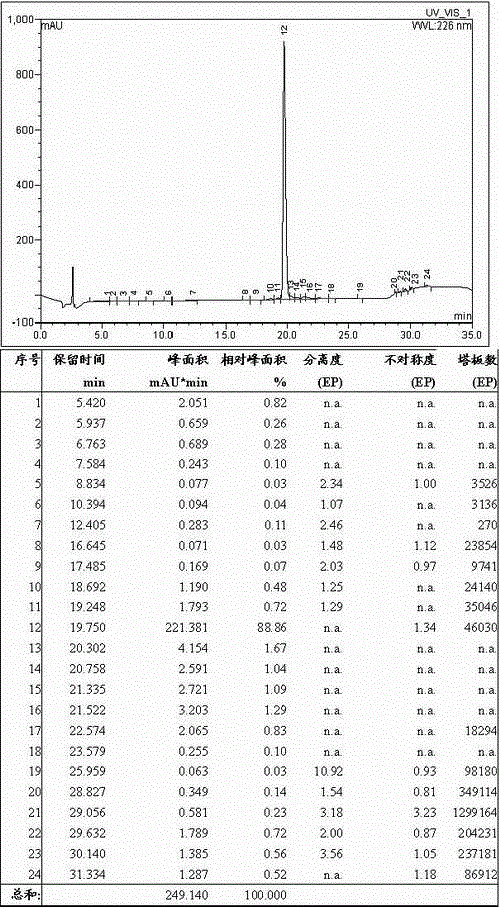
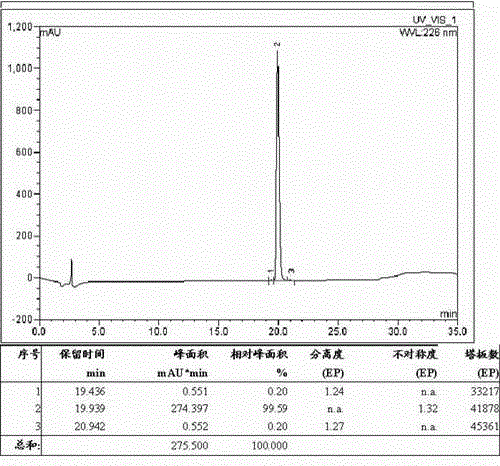
[0092] Weigh 50.00g of fully protected cetrorelix crude peptide resin, add it into a 1000mL three-necked round-bottomed flask, and use TFA, Tis and H with a volume ratio of 95:2.5:2.5 2 Prepare 500 mL of the lysate composed of O, add the lysate to the above resin, react at room temperature for 2 hours, filter, wash the cleaved resin with a small amount of TFA for 3 times, combine the filtrate, concentrate, and add the concentrated liquid to glacial ether for precipitation 1 hour, centrifugal, anhydrous ether centrifugal washing 6 times, vacuum-drying, obtains cetrorelix crude peptide 145.00g, and its HPLC spectrogram is as follows figure 2 As shown, the HPLC purity was 88.86%, and the crude peptide yield was 78%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com