Preparation method of high-purity boron trichloride-11
A technology of high-purity boron trichloride and boron trichloride, which is applied in the field of preparation of high-purity boron trichloride-11, can solve the problems of semiconductor device performance impact, machine crash, and impact on the operating speed of electronic equipment, and achieve Improve the anti-interference and anti-radiation performance, the effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] A preparation method of high-purity boron trichloride-11, comprising the following steps:
[0036] (i) Preprocessing
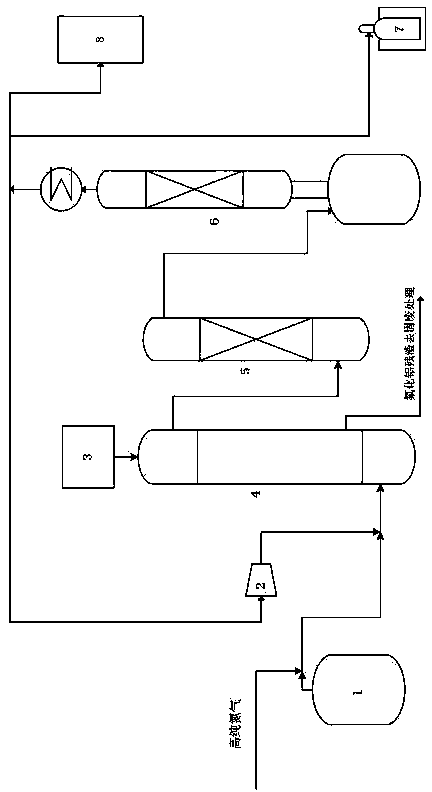
[0037] Add the aluminum chloride in the feeder 3 into the reactor 4 through the hopper, heat the reactor 4 to 90°C~100°C after passing the leak test, and feed high-purity nitrogen into the reactor 4 to replace the air therein until The water content of the discharged nitrogen is less than 1ppm;
[0038] (ii) Reaction of aluminum chloride and boron trifluoride-11 to synthesize boron trichloride-11
[0039] Pass the high-abundance boron trifluoride-11 in the raw material storage tank 1 into the reactor 4 to react with the pretreated aluminum chloride, the reaction temperature is 100-300°C, the reaction pressure is 0.05-2.0MPa, and after the reaction, Boron trichloride-11 crude product, including unreacted boron trifluoride-11 and hydrogen fluoride, hydrogen chloride, air impurities and entrained aluminum chloride and aluminum fluoride particles in the b...
Embodiment 1
[0055] (i) Preprocessing
[0056] Add the aluminum chloride in the feeder 3 into the reactor 4 through the hopper, heat the reactor 4 to 90°C after the leak test is qualified, and feed high-purity nitrogen into the reactor 4 to replace the air until the content of the discharged nitrogen is The amount of water is less than 1ppm;
[0057] (ii) Reaction of aluminum chloride and boron trifluoride-11 to synthesize boron trichloride-11
[0058]Pass the high-abundance boron trifluoride-11 in the raw material storage tank 1 into the reactor 4 to react with the pretreated aluminum chloride, the reaction temperature is 100°C, the reaction pressure is 0.05MPa, and boron trichloride is obtained after the reaction -11 crude product, including unreacted boron trifluoride-11 and hydrogen fluoride, hydrogen chloride, air impurities and entrained aluminum chloride and aluminum fluoride particles in the boron trichloride-11 crude product;
[0059] (iii) Filtration
[0060] The boron trichlo...
Embodiment 2
[0071] (i) Preprocessing
[0072] Add the aluminum chloride in the feeder 3 to the reactor 4 through the hopper, heat the reactor 4 to 95°C after the leak test is qualified, and feed high-purity nitrogen into the reactor 4 to replace the air until the discharged nitrogen contains The amount of water is less than 1ppm;
[0073] (ii) Reaction of aluminum chloride and boron trifluoride-11 to synthesize boron trichloride-11
[0074] Pass the high-abundance boron trifluoride-11 in the raw material storage tank 1 into the reactor 4 to react with the pretreated aluminum chloride, the reaction temperature is 150°C, the reaction pressure is 0.5MPa, and boron trichloride is obtained after the reaction -11 crude product, including unreacted boron trifluoride-11 and hydrogen fluoride, hydrogen chloride, air impurities and entrained aluminum chloride and aluminum fluoride particles in the boron trichloride-11 crude product;
[0075] The reaction formula is as follows:
[0076] AlCl 3 +...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com