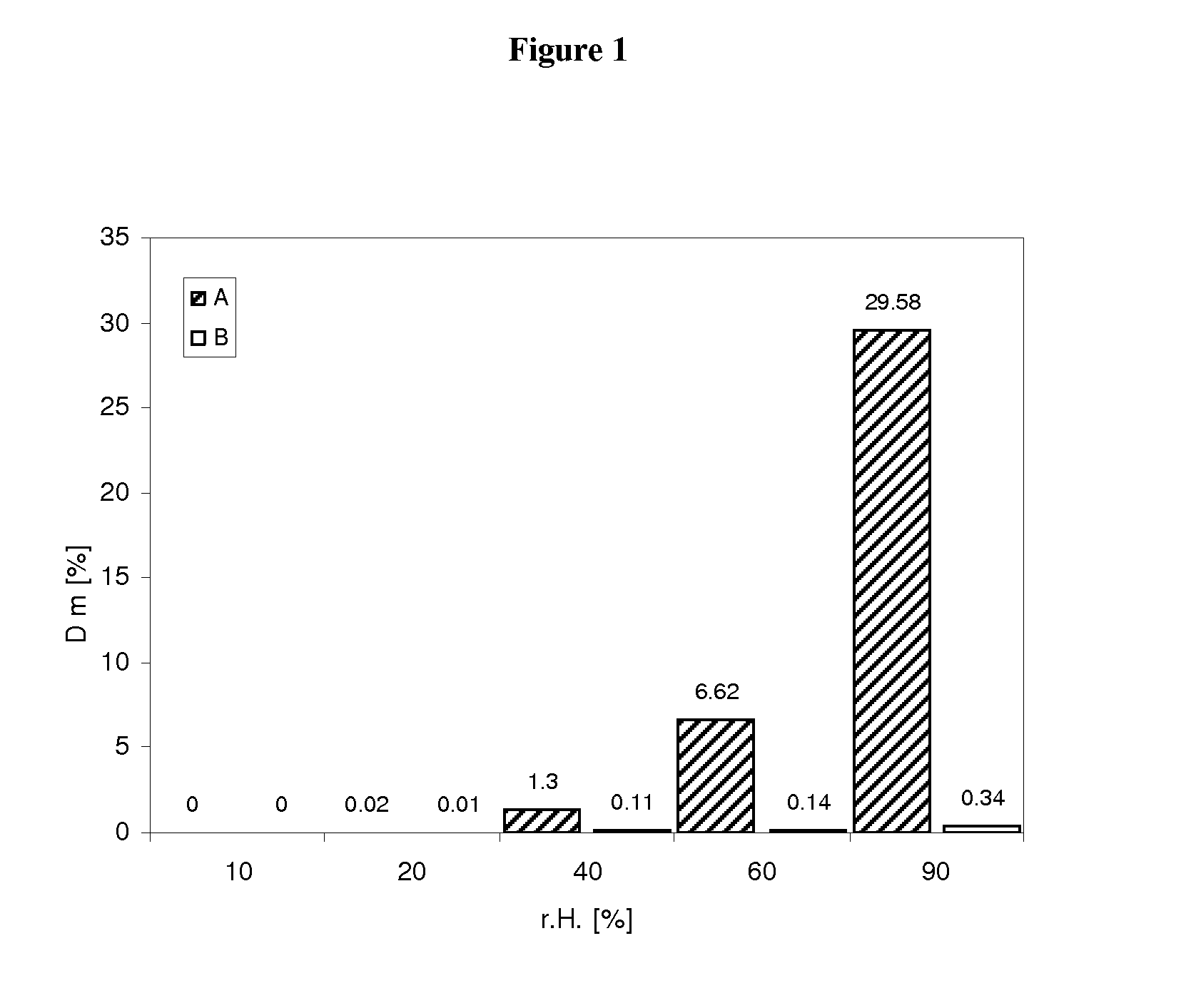
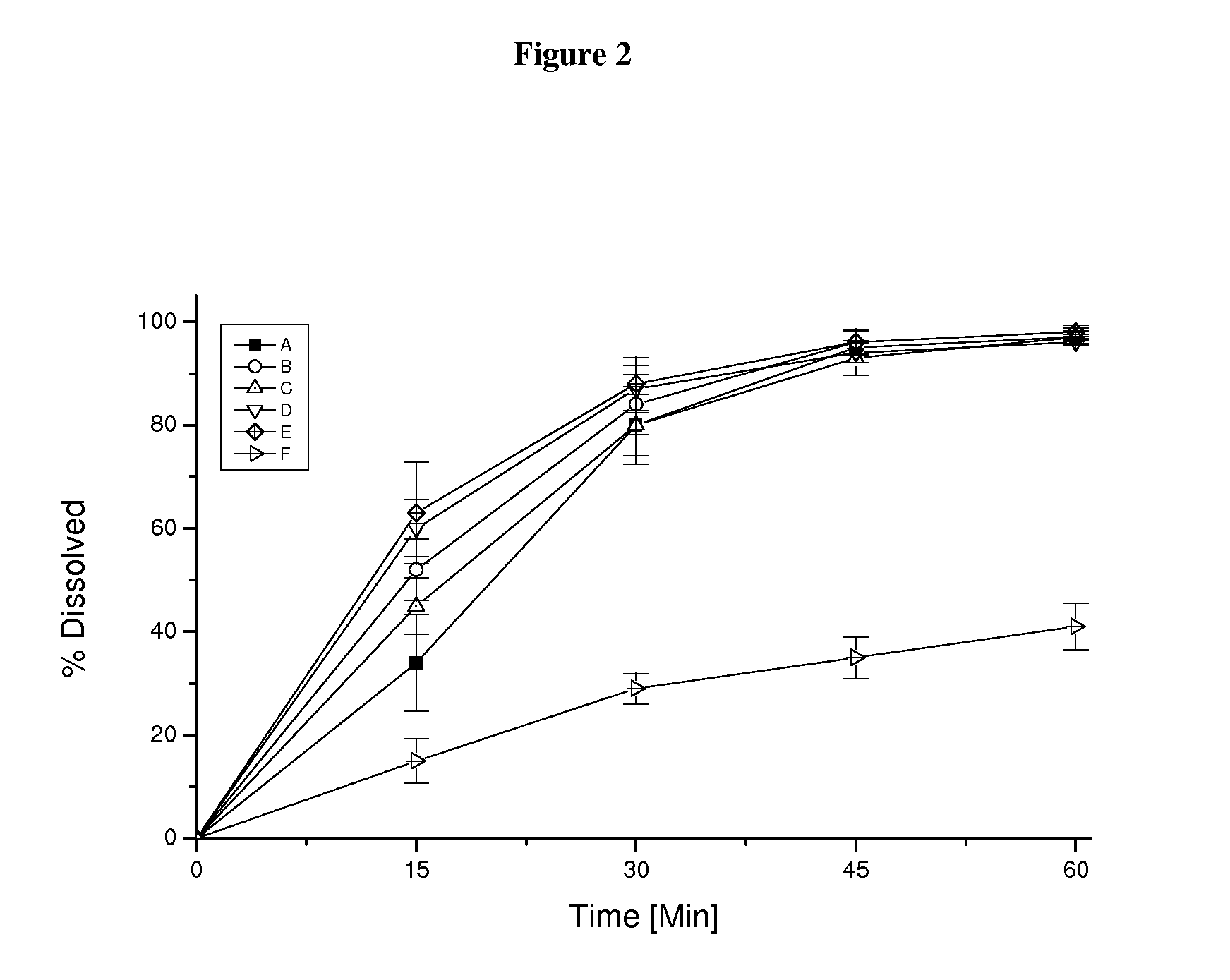
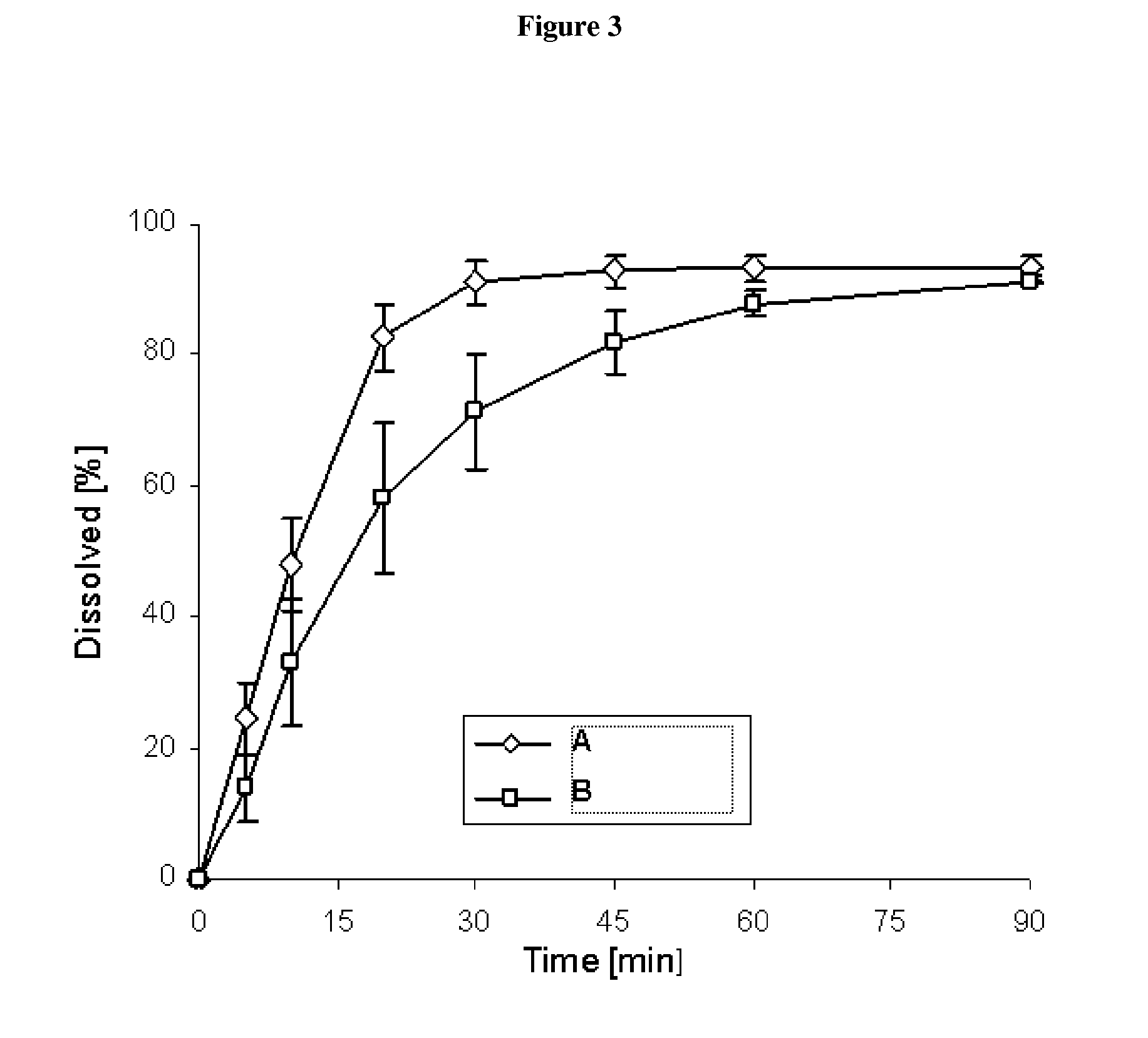
Capsule pharmaceutical dosage form comprising a suspension formulation of an indolinone derivative
a technology of indolinone derivative and dosage form, which is applied in the direction of packaging foodstuffs, immunodeficiency disorders, packaged goods, etc., can solve the problems of drug insufficient soluble in any lipid formulation, difficult to formulate into common administration forms, and limited choice of formulations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Lipid Based Carrier System
[0075]
FormulationABCIngredients[%]*Active Substance43.4843.4843.48Triglycerides,28.7037.8338.045Medium-ChainHard fat27.3918.2618.26Lecithin0.430.430.215*slight deviations of the quantities towards 100 percent may be caused by rounding errors
example 2
Lipid Based Carrier System with Additional Surfactant
[0076]
Ingredients[%]*Active Substance42.19Triglycerides,41.77Medium-ChainHard fat12.66Cremophor RH402.95Lecithin0.42*slight deviations of the quantities towards 100 percent may be caused by rounding errors
example 3
Hydrophilic Carrier System
[0077]
Ingredients[%]*Active Substance31.75Glycerol 85%3.17Purified Water4.76Macrogol 60058.10Macrogol 40002.22*slight deviations of the quantities towards 100 percent may be caused by rounding errors
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
| weight % | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com