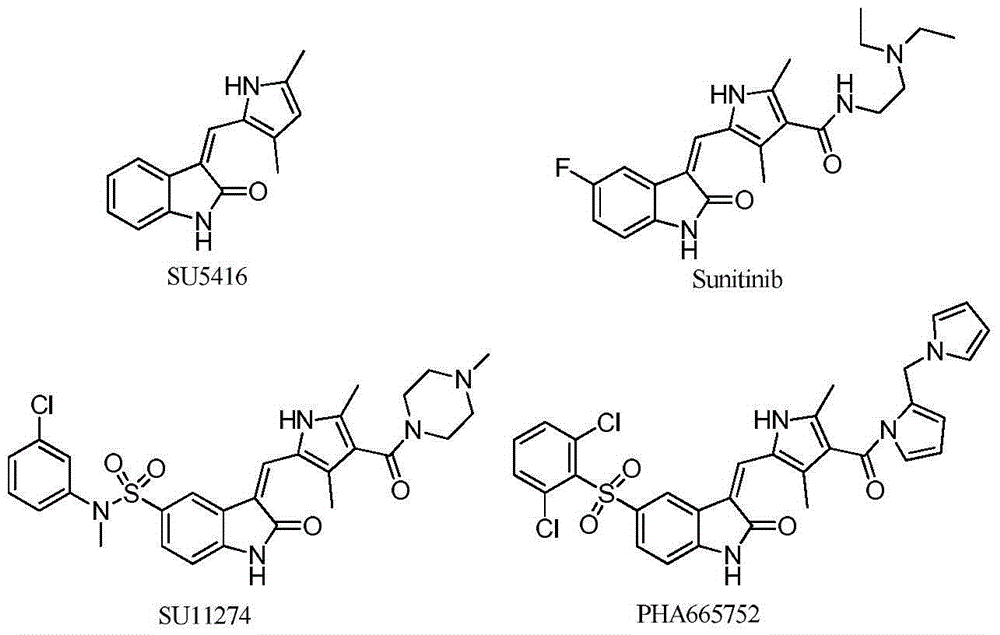
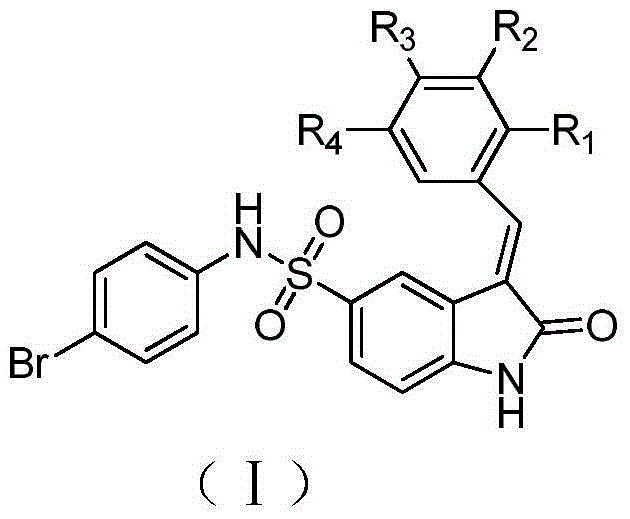
2-indolinone derivatives, preparation and applications thereof
A technology of indolinone and its derivatives, which is applied in the field of 2-indolinone derivatives and their pharmacological activity and pharmaceutical use, can solve the problem of no 2-indolinone derivative pharmaceutical composition, etc., and achieve good anti-tumor and Effect of anti-angiogenic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
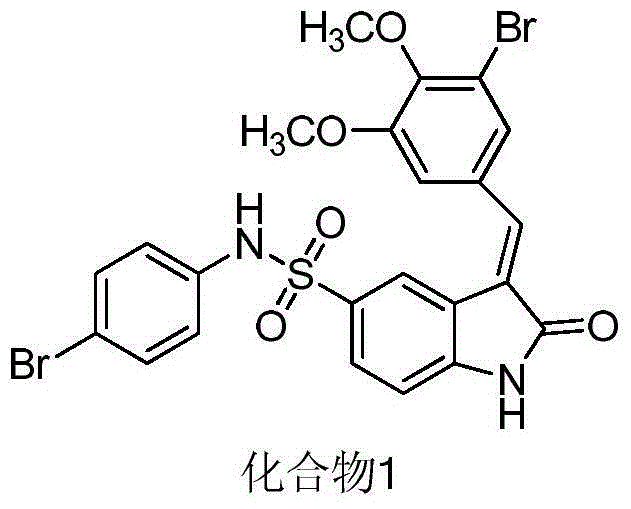
[0037] Preparation of 3-(3-bromo-4,5-dimethoxyphenylmethylene)-N-(4-bromophenyl)-2-indolinone-5-sulfonamide (compound 1):
[0038](1) Carefully add 10 ml of chlorosulfonic acid into a 50 ml three-neck flask, add 10 mmoles of 2-indolinone under ice bath, stir at room temperature, react at 65 degrees Celsius for 1 hour, cool to room temperature, add to In ice water, filter and dry to obtain a pink intermediate;
[0039] (2) Dissolve 1.15 grams of the intermediate in 50 milliliters of tetrahydrofuran, then add 1.8 grams of 4-bromoaniline, heat to 80 degrees Celsius for 3 hours, evaporate THF under reduced pressure, and add 25 milliliters of PH=3 to the residual solid Dilute hydrochloric acid, stir, and filter with suction to obtain a pink solid;
[0040] (3), then dissolve it in 50 ml of absolute ethanol, add 5 mmoles of 3-bromo-4,5-dimethoxybenzaldehyde, add dropwise 100 microliters of piperidine as a catalyst, and reflux for 3 hours , cooled, filtered, washed with water and d...
Embodiment 2
[0042] 3-(2,3-dibromo-4,5-dimethoxyphenylmethylene)-N-(4-bromophenyl)-2-indolone-5-sulfonamide (compound 2) preparation:
[0043] The preparation method of compound 2 is similar to the preparation method of compound 1, and its difference from Example 1 is that the raw material 3-bromo-4,5-dimethoxybenzaldehyde is replaced by 2,3-dibromo-4,5 -Dimethoxybenzaldehyde, compound 2 was prepared, yellow solid, yield 83%, 1HNMR (DMSO-d6, 500MHz, ppm): δ11.16 (s, 1H), 10.24 (s, 1H), 7.66 ( s,1H),7.60(d,1H,J=8.5Hz),7.57(s,1H),7.52(s,1H),7.31(d,2H,J=8.5Hz),6.99(d,1H,J =8.5Hz),6.90(d,2H,J=8.5Hz),3.86(s,3H),3.82(s,3H); 13CNMR(DMSO-d6,125MHz,ppm):δ168.5,152.8,148.6,147.2, 137.5, 136.8, 132.4(2C), 132.2, 131.7, 130.0, 128.0, 122.2(2C), 122.1, 121.7, 121.2, 117.0, 116.6, 113.9, 110.9, 60.9, 60.7; ESIMS: m / z668[M-SIMH]-HRE :calcforC23H16Br3N2O5S[M-H]-668.8334,found668.8335.
Embodiment 3
[0045] Determination of the inhibitory activity of 2-indolinone derivatives on various tumor cells:
[0046] The cytotoxicity of synthetic derivatives to human tumor cells cultured in vitro was detected by the commonly used tetrazolium salt (MTT) method. Cell lines selected for anti-tumor experiments in vitro: human lung cancer cell A549, human liver cancer cell Bel7402, human liver cancer cell HepG2, human cervical cancer cell Hela, human colon cancer cell HCT116, etc. Determination method: take the cells in the logarithmic growth phase, inoculate the cell suspension into a 96-well plate, so that the number of cells per well is 3×103, at 37°C, 100% relative humidity, containing 5% CO2 by volume, 95 % air incubator pre-cultivation for 24h, and then add drugs. In addition, each concentration of compound 1 and 2 with a concentration of 1.25, 2.5, 5.0, 10.0, and 20.0 μg / ml was set as a negative control (equal concentration of DMSO) and a blank background (without adding cells), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com