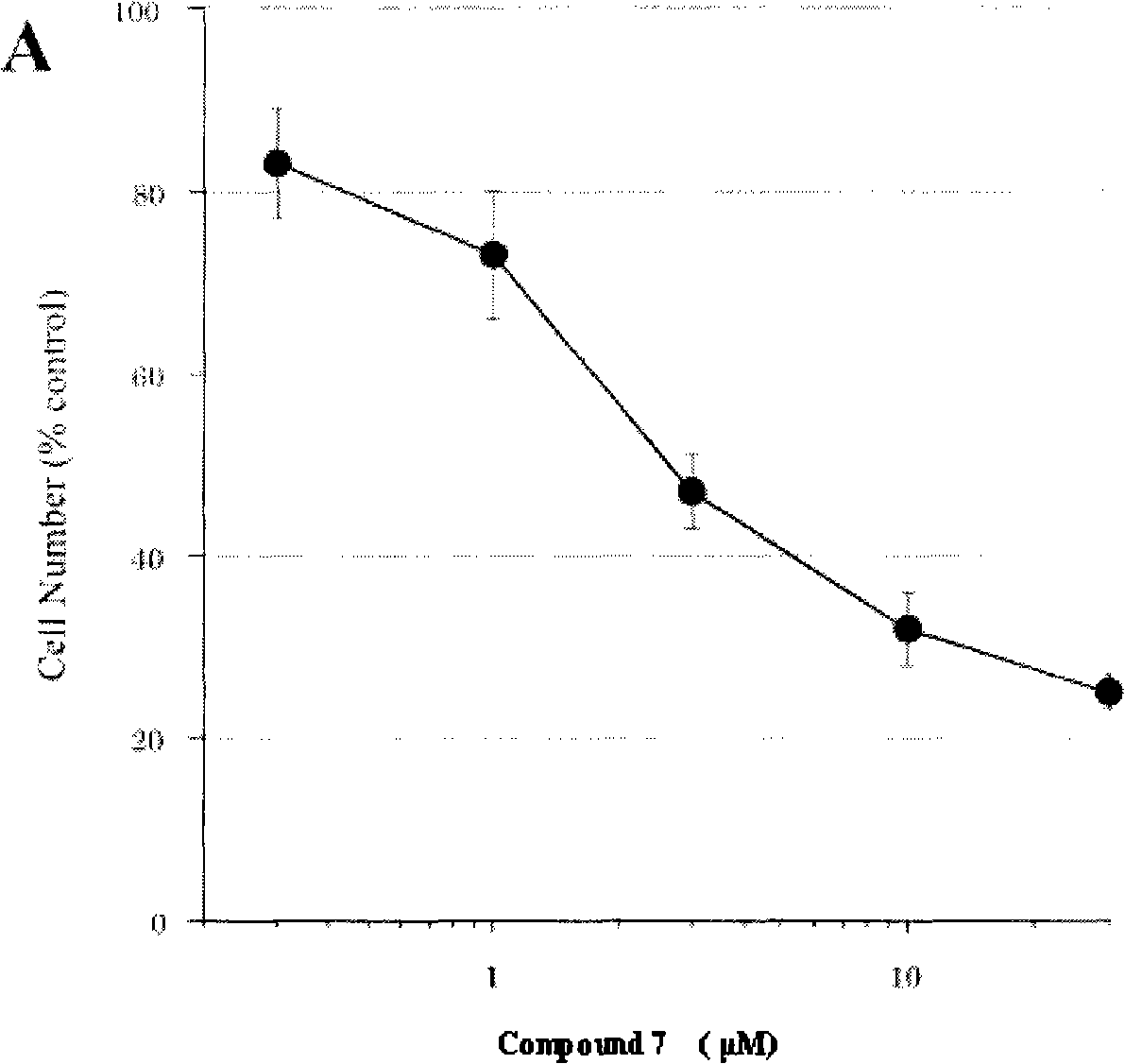
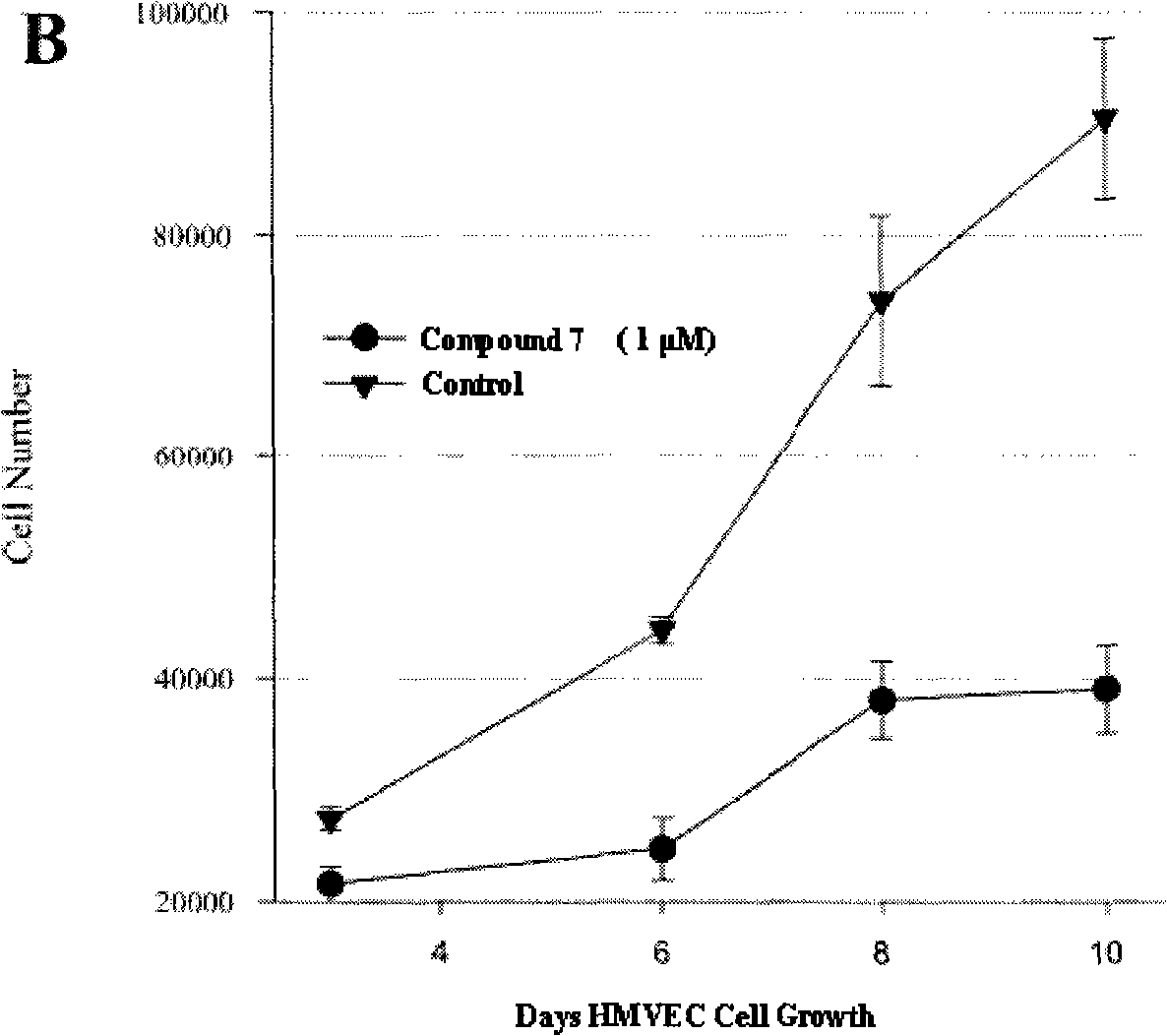
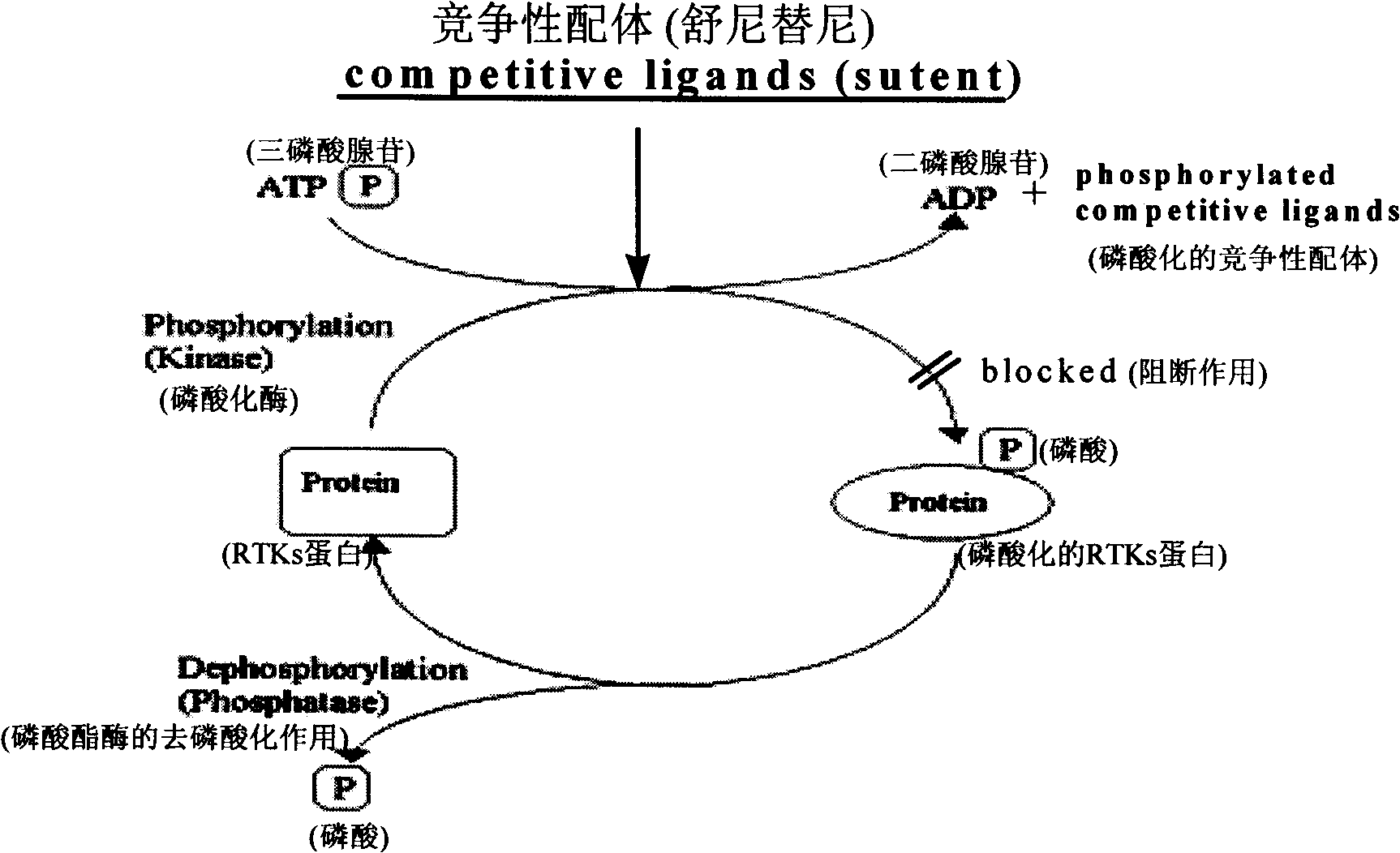
Substituted 2-indoline ketone derivatives as well as preparation method and uses thereof
A technology of indolinone and its derivatives, which is applied in the field of substituted 2-indolinone derivatives, can solve the problem that cancer cells cannot be completely eliminated, and achieve the effects of high yield of finished products, improved solubility, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0042] One. The preparation of the compound of the present invention
[0043] Synthesis of compound 3:
[0044] Compound 1 (1.51 g, 0.01 mol) and 50 ml of hexamethyldisilamine were mixed and refluxed for 1 hour, the solvent was evaporated under reduced pressure, and the residue was dissolved in 50 ml of anhydrous acetonitrile, and compound 2 (3.18 g, 0.01 mol) and stirred evenly. Add trimethylsilyl trifluoromethanesulfonate (0.7mL, 0.03mol) at 0°C. The mixture was heated to reflux for 1 hour, cooled to room temperature, and 20 ml of saturated sodium bicarbonate solution was slowly added under cooling , after stirring, extract with dichloromethane (3 × 30mL), combine the extracts, wash once with brine, and dry over anhydrous sodium sulfate. Filter, evaporate to dryness, and the residual silica gel column chromatography (eluent: ethyl acetate-n-hexane Alkane 1:3) to obtain compound 3 as an oily liquid (3.1 g, 76%). ESIMS: m / z 410 (M+1).
[0045] Synthesis of compound 5:
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com