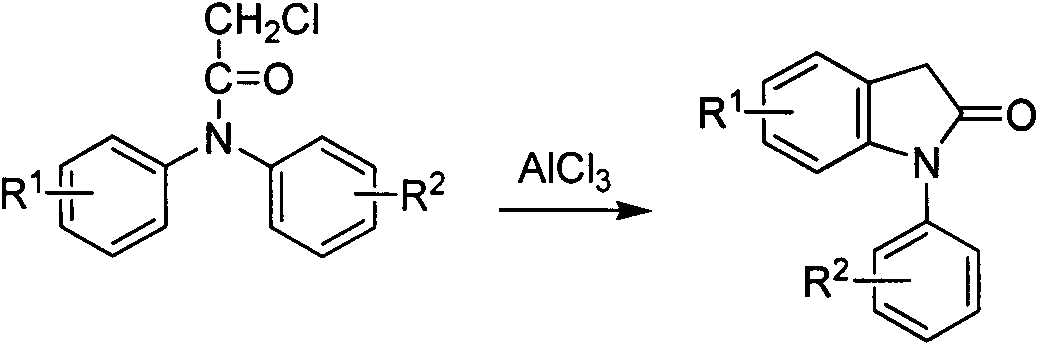
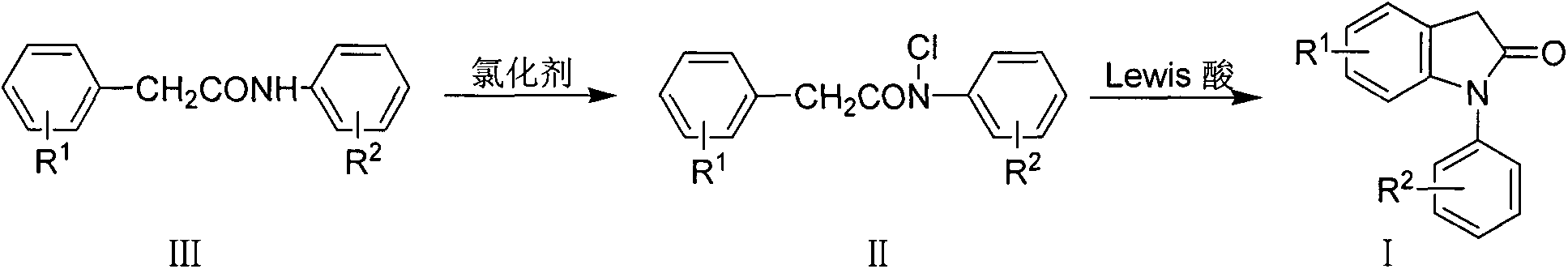
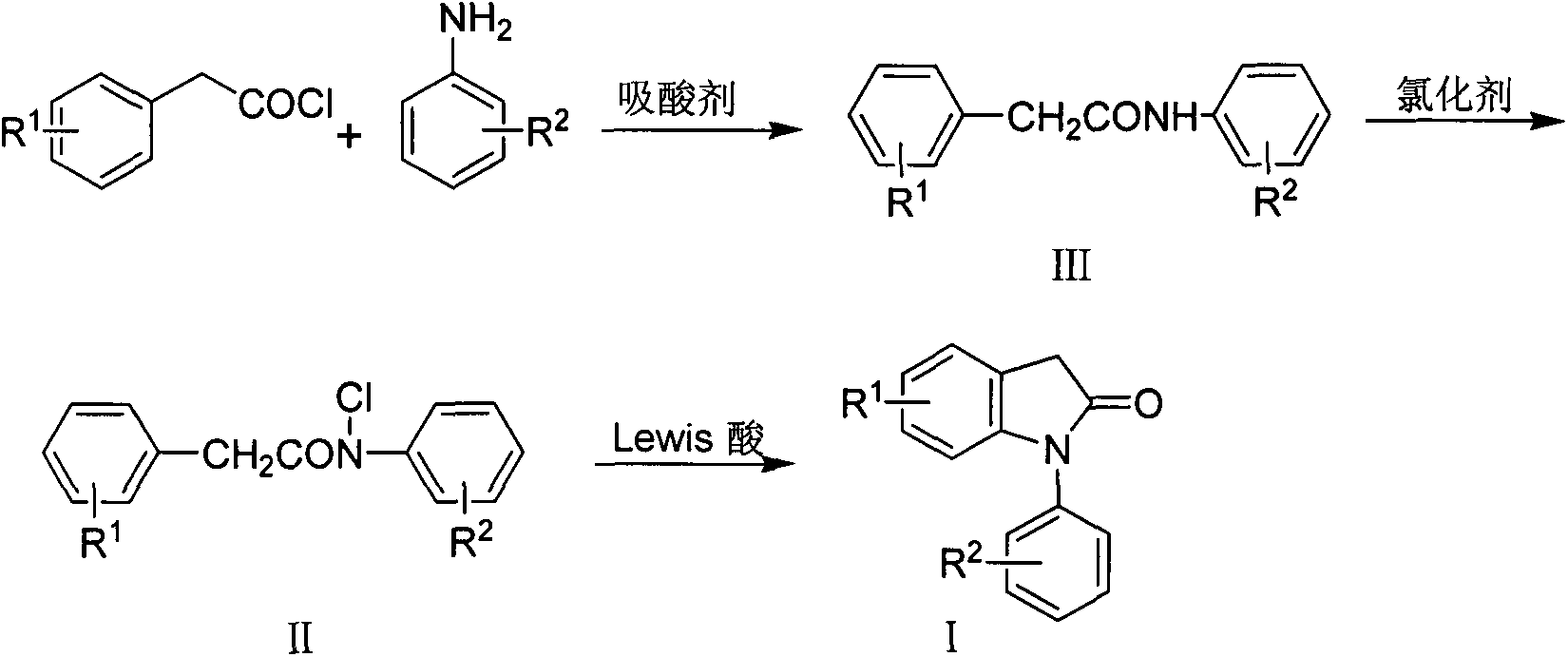
Preparation method for 1-aryl-2-indolinone derivatives
A technology of indolinone and its derivatives, applied in the direction of organic chemistry, etc., can solve the problems of expensive raw materials, few reports on synthetic methods, high reaction temperature, etc., and achieve the effect of cheap raw materials, low cost and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] (a) the preparation of N-(2,6-dichlorophenyl) phenylacetamide (III-a)
[0021] method one:
[0022] Add 16.20g (0.1mol) of 2,6-dichloroaniline and 9.49g (0.12mol) of pyridine to a 250ml three-neck flask equipped with a thermometer, constant pressure dropping funnel, reflux condenser and anhydrous calcium chloride drying tube. and 100ml acetone. After stirring, cooling in an ice-water bath, 15.45 g (0.1 mol) of phenylacetyl chloride was slowly added dropwise. After the addition was complete, the ice-water bath was removed. Heating, reflux 4h. Stop responding.
[0023] The reaction solution was poured into 250ml of 1.5mol / L hydrochloric acid solution while hot, and a large amount of yellow blocky solid appeared. The solid was filtered off and washed successively with 100ml×2 3mol / L hydrochloric acid solution, 100ml×2 1mol / L NaOH solution, and 100ml×2 deionized water. After drying, a yellow solid was obtained. 26.50 g of white needle-like crystals were obtained by r...
Embodiment 2
[0062] (a) Preparation of N-(4-chlorophenyl) phenylacetamide (III-b)
[0063] Using p-chloroaniline and phenylacetyl chloride as raw materials, N-(4-chlorophenyl)phenylacetamide (III-b) was prepared according to the first method of Example 1 (a), and finally 23.95 g of white needle-like crystals were obtained. The yield 96.3%. Melting point: 161.5-163.9°C
[0064] (b) Preparation of N-chloro-N-(4-chlorophenyl) phenylacetamide (II-b)
[0065] Taking N-(4-chlorophenyl) phenylacetamide (III-b) as raw material, prepare N-chloro-N-(4-chlorophenyl) phenylacetamide (II- b) Finally, 27.98 g of light yellow solid was obtained, and the yield was about 100%.
[0066] (c) Preparation of 1-(4-chlorophenyl)-2-indolinone (I-b)
[0067] Using N-chloro-N-(4-chlorophenyl)phenylacetamide (II-b) as raw material, prepare 1-(4-chlorophenyl)-2-dihydroindoline according to method 1 of Example 1(c) Indolin ketone (I-b), 17.61 g of a light yellow solid was finally obtained, with a yield of 72.3%. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com