Method for preparing diclofenac sodium
A technology of diclofenac and dichlorophenyl is applied in the field of preparation of diclofenac, can solve the problems of complicated operation, expensive raw materials, toxic raw materials and the like, and achieves the effects of simple operation, cheap raw materials, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
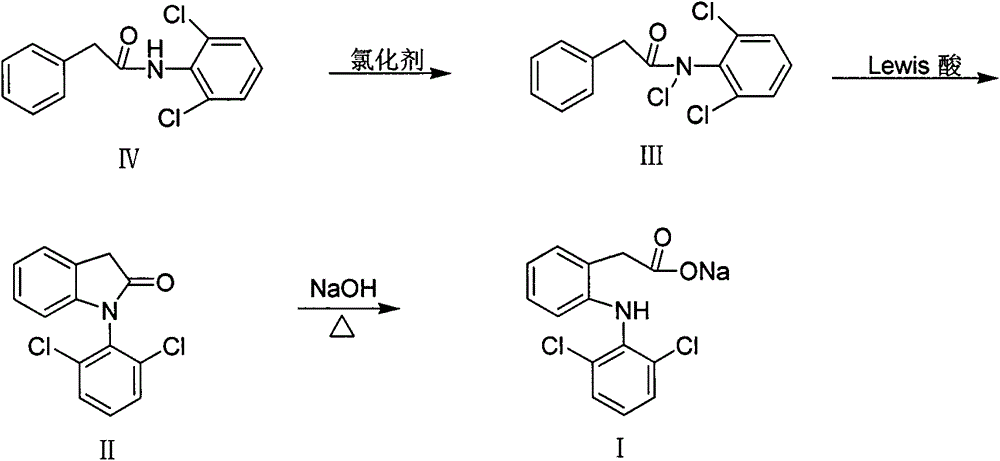
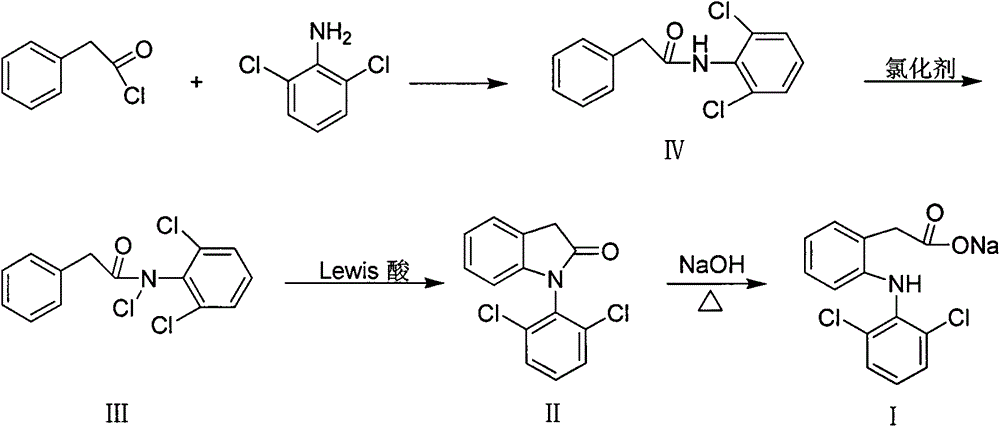
[0024] The total reaction equation of the preparation method of diclofenac of the present invention is as follows:
[0025]
Embodiment 1
[0027] (a) the preparation of N-(2,6-dichlorophenyl) phenylacetamide (IV)
[0028] Add 16.20g (0.1mol) of 2,6-dichloroaniline and 9.49g (0.12mol) of pyridine to a 250ml three-necked flask equipped with a thermometer, a constant pressure dropping funnel, a reflux condenser and anhydrous calcium chloride drying tube. and 100ml acetone. After stirring, cooling in an ice-water bath, 15.45 g (0.1 mol) of phenylacetyl chloride was slowly added dropwise. After the addition was complete, the ice-water bath was removed. Heating, reflux 4h. Stop responding.
[0029] The reaction solution was poured into 250ml of 1.5mol / L hydrochloric acid solution while hot, and a large amount of yellow blocky solids appeared. The solid was filtered off and washed successively with 100ml×2 3mol / L hydrochloric acid solution, 100ml×2 1mol / L NaOH solution, and 100ml×2 deionized water. After drying, a yellow solid was obtained. 26.50 g of white needle-like crystals were obtained by recrystallization f...
Embodiment 2
[0039] (a) the preparation of N-(2,6-dichlorophenyl) phenylacetamide (IV)
[0040] Add 16.20g (0.1mol) of 2,6-dichloroaniline, 12.12g (0.12mol) of three Ethylamine and 150ml of dichloromethane. Stir and cool in an ice-water bath, slowly add 15.45 g (0.1 mol) of phenylacetyl chloride dropwise, and control the temperature of the reaction solution not to exceed 25°C. After the addition was complete, the ice-water bath was removed. Heating, reflux 6h. Stop responding.
[0041] After the reaction was completed, 50 ml of deionized water was added to the reaction solution, and a large amount of white solids were precipitated. Filter and wash the filter cake with 100ml×3 deionized water. The filtrate was separated and the organic phase was separated. The organic phase was washed successively with 50ml×2 10% NaOH solution, 50ml×2 3mol / L hydrochloric acid solution, and 250ml×2 deionized water. The organic phase was dried over anhydrous magnesium sulfate, and the solvent was disti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com