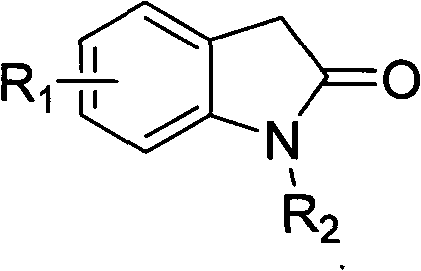
Method for preparing substituted 2-indolinone compound
A technology of ketone compounds and indole, which is applied in the field of preparation of substituted indol-2-one compounds, can solve the problems of high reaction temperature, low reaction yield, and difficulty in obtaining it, and achieve convenient and simple post-processing Convenience and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment one: the preparation of 5-bromoindol-2-one:
[0024] Under the protection of nitrogen, add 100ml trifluoroacetic acid and 34.8g (0.3mol) triethylsilane in the 250ml reaction bottle, after stirring evenly, add 22.6g (0.1mol) 5-bromoisatin in batches at room temperature, after adding The reaction was stirred for 12 hr at room temperature. Concentrate under reduced pressure to remove trifluoroacetic acid, then add 50ml of diethyl ether, stir for 1 hr, filter, wash with diethyl ether, and drain to obtain an off-white solid. After drying, 19.5g of 5-bromoindol-2-one is obtained, with a yield of 91.9%, mp =217~219°C, -cESI=212.05, 1HNMR-δ((CD 3 ) 2 SO) / 300MHz): 10.38(s, 1H), 7.44-7.31(m, 2H), 6.74(d, J=8.0Hz, 1H), 6.27(d, J=7.7Hz, 1H), 4.86(d, J=7.6Hz, 1H).
Embodiment 2
[0025] Example 2: Preparation of 5-fluoroindol-2-one:
[0026] Take 16.5g (0.1mol) of 5-fluoroisatin and prepare according to the method of Example 1 to obtain 13.6g of 5-fluoroindol-2-one, the yield is 90.3%, mp=141~143°C, -cESI=150.08, 1H NMR-δ((CD 3)2 SO) / 300MHz): 10.25(s, 1H), 7.12(dd, J=8.1, 2.5Hz, 1H), 7.08-6.96(m, 1H), 6.76(dd, J=8.4, 4.3Hz, 1H), 6.26 (d, J=7.6Hz, 1H), 4.84 (d, J=7.6Hz, 1H).
Embodiment 3
[0027] Embodiment three: the preparation of 5-chloroindol-2-one:
[0028] Take 18.2g (0.1mol) of 5-chloroisatin and prepare it according to the method of Example 1 to obtain 15.6g of 5-chloroindol-2-one, the yield is 92.7%, mp=196~198°C, -cESI=166.87, 1H NMR-δ((CD 3)2 SO) / 300MHz): 10.35(s, 1H), 7.30-7.21(m, 2H), 6.77(dd, J=8.1, 4.9Hz, 1H), 6.27(d, J=7.6Hz, 1H), 4.86( d, J=7.6Hz, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com