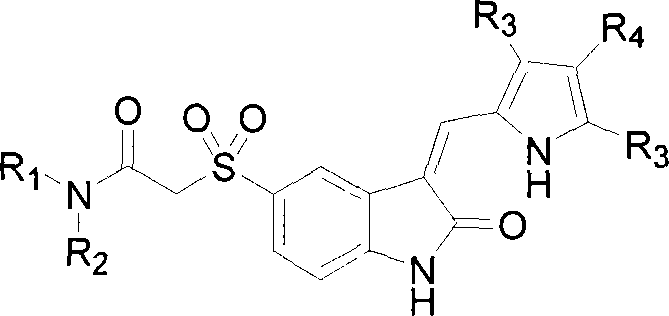
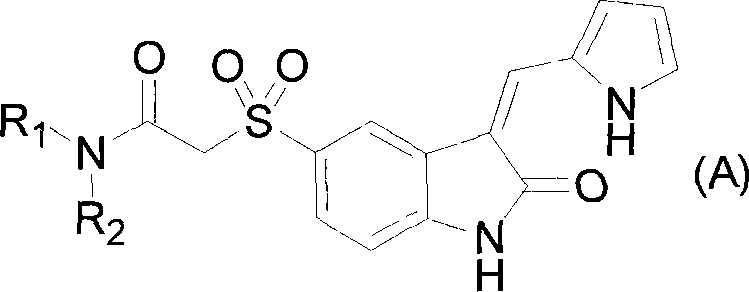
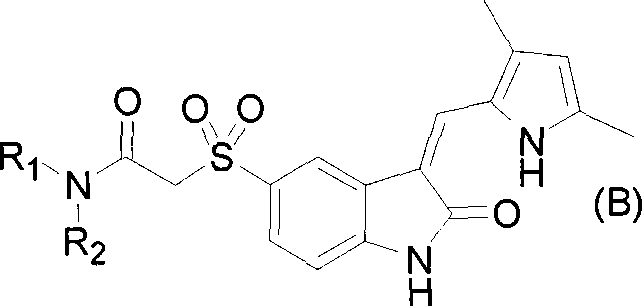
2-indole ketone compound, preparation and uses thereof
A technology of indolinone and compound, applied in the field of chemical synthesis and application, can solve the problem of unsatisfactory activity concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] 5-Chlorosulfonyl-2-indolinone
[0085] 2g of compound 3 was added in batches to 5ml of chlorosulfonic acid under ice cooling and stirred, and the system became black and viscous. After the addition, the system was slowly warmed up to 60° C. and stirred for 2 hours. After stopping heating, cool to room temperature. The reaction solution was poured into 200 g of crushed ice and continued to stir for 2 hours. Suction filtration, washing with water to neutrality, and vacuum drying gave 2.3 g of light yellow powder (4), with a yield of about 66%. .
Embodiment 2
[0087] 5-Hydroxysulfinyl-indol-2-one
[0088] 7.2gNa 2 HPO 4 .H 2 O and 5.0gNa 2 SO 3 After it was completely dissolved in 40ml of water at 30°C, 4.6g of compound 4 was added in batches and stirred. The temperature was raised to 60° C. and stirred for 4 hours. Remove from heat and cool to room temperature. Cooled in an ice bath with 9N H 2 SO 4 Adjust the pH to 1-2, and a large amount of light yellow solid precipitates. Suction filtration and vacuum drying yielded 2.83 g of light yellow compound 5, which was purified by beating with PE, washed with methanol, and concentrated with a yield of about 72%.
Embodiment 3
[0090] 5-Carboxymethylsulfonyl-indol-2-one
[0091] Dissolve 3g of compound 5 in 20ml of water, 5.8g of chloroacetic acid in 20ml of water, and adjust the pH to 7-8 with 30% NaOH aqueous solution respectively. Mix the two, stir, heat up to 95°C, and control the reaction system with 0.5N NaOH aqueous solution The pH value is between 7-8, and the reaction takes 4 hours. Remove from heat and cool to room temperature. The pH was adjusted to 1 with 3N HCl solution, and a large amount of solids precipitated out as pale yellow. Suction filtration and drying gave 2.4 g of light yellow powder (6), which was purified by beating with PE, with a yield of about 62%. 1 HNMR (DMSO-d 6 ) (ppm): 11.2 (1H, s), 10.85 (1H, d), 7.71 (2H, t), 6.98 (1H, d), 4.33 (2H, s), 1.89 (1H, s).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com