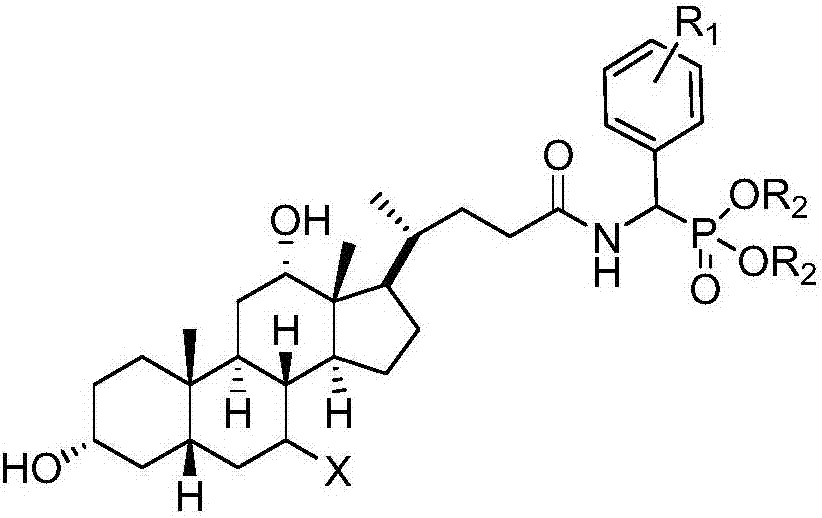
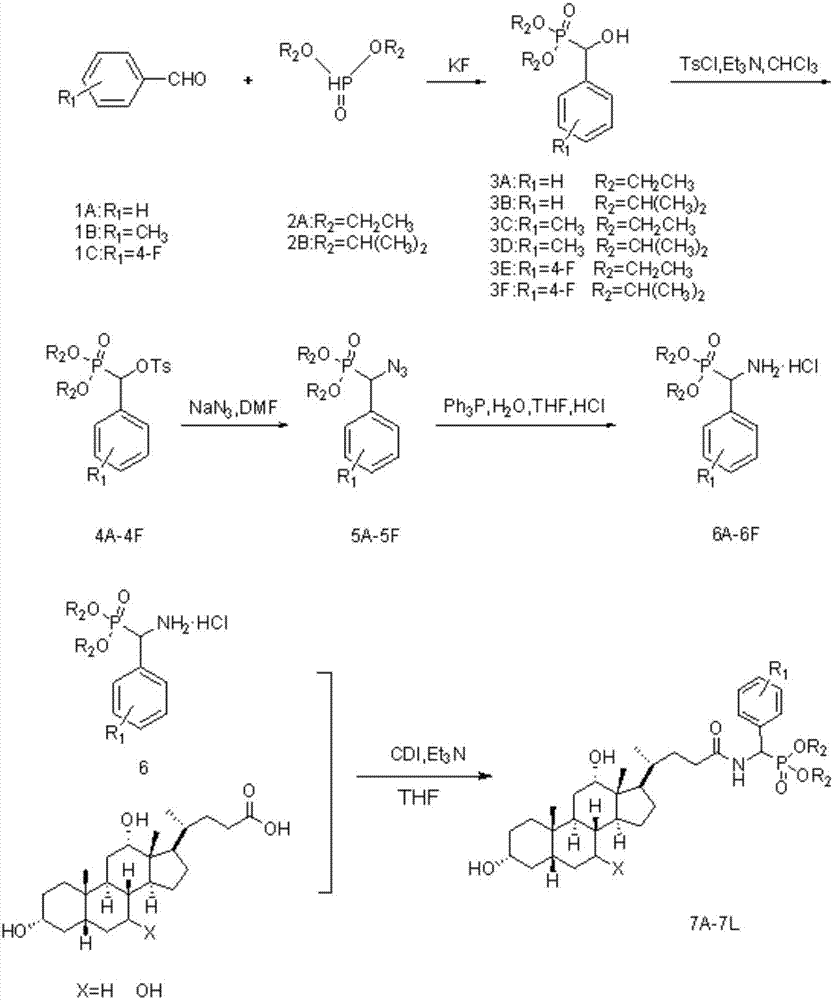
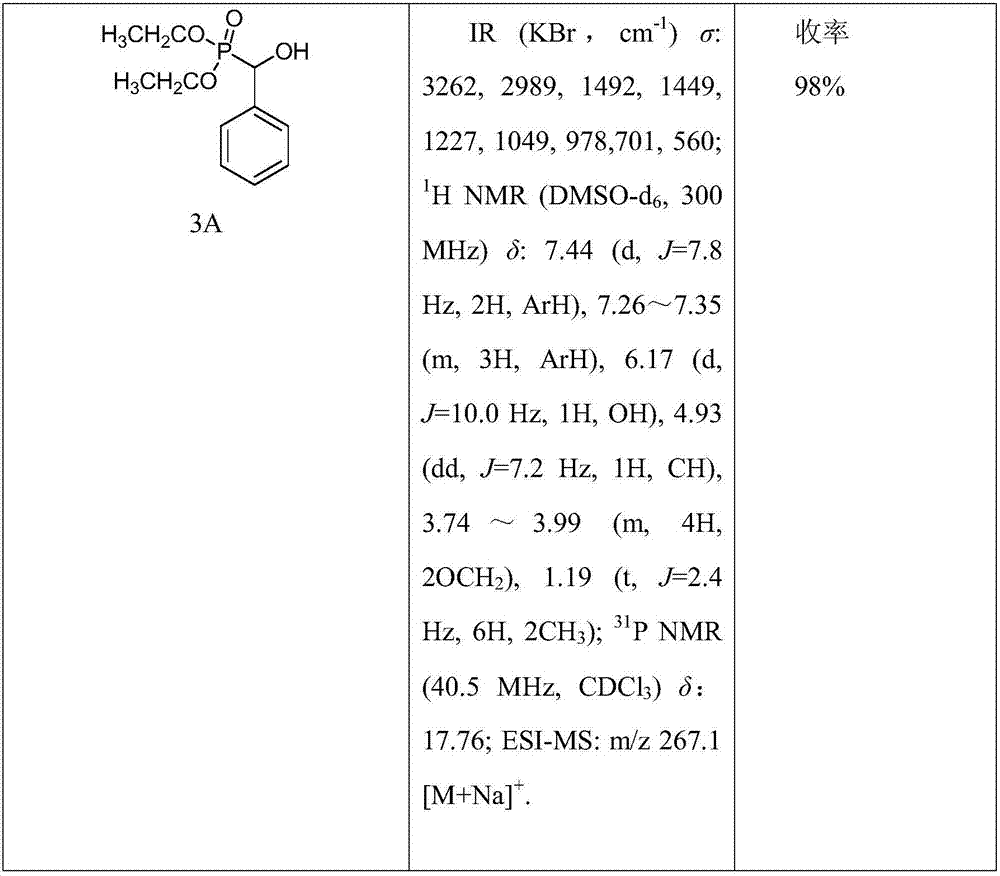
Cholic acid-α-aminophosphonate derivative and method for synthesizing the same
A technology of aminophosphonates and derivatives is applied in the field of novel cholic acid-α-aminophosphonate compounds and their preparation, and can solve the problems such as cholic acid-α-aminophosphonate derivatives that have not yet been seen, Achieve the effects of excellent target selectivity, high yield, and excellent anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Synthesis of compound 3: Add 10mmol of 2A compound (or 2B compound) in a 50mL round bottom flask, then slowly drop 12mmol of 1A compound (or 1B, 1C compound), after stirring evenly, add 1.15g of Anhydrous Potassium Fluoride. Stir vigorously at room temperature until all the liquid in the flask turns into a white solid, add 30 mL of dichloromethane to dissolve it, filter to remove potassium fluoride, distill under reduced pressure to spin the filtrate to dryness, and recrystallize to obtain intermediate 3A (or 3B~ 3J).
[0027] (2) Synthesis of compound 4: Add 40mL of dry chloroform to a 100mL round bottom flask, add 10mmol of 3A compound or (3B~3F compound), stir to dissolve, then drop in 15mmol of triethylamine as an acid-binding 12mmol of p-toluenesulfonyl chloride was dissolved in 20mL of dry chloroform, slowly added dropwise to the flask, reacted at 0°C for 2h, and finally rose to room temperature, and TLC monitored the reaction progress. After the reaction, t...
Embodiment 2
[0042] Synthesis of compounds 7A-7L: Dissolve 5 mmol of deoxycholic acid (or cholic acid) in 40 mL of dry tetrahydrofuran, add 10 mmol of N,N-carbonyldiimidazole (CDI), stir for 30 min, and then add 6 mmol of compound 6A (or 6B~6F), add 10 mmol of triethylamine dropwise, and react at 45°C. The reaction process was monitored by TLC. After the reaction was completed, the solvent was removed under reduced pressure, dissolved in ethyl acetate, washed three times with dilute hydrochloric acid, saturated sodium bicarbonate solution, and saturated brine successively, and the organic phase was dried with anhydrous sodium sulfate, filtered, and reduced The solvent was removed under pressure, and column chromatography separated V (ethyl acetate): V (petroleum ether): V (dichloromethane) = 1: 5: 0.1 to obtain white solid compound 7A (or 7B-7L).
[0043] The chemical structure, infrared, and NMR data of some preferred compounds synthesized by the present invention are shown in the followi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com