Halogenated pyrrole-substituted 2-indolinone salt and preparation method and application thereof
A technology of indolinone salt and halogenated pyrrole, which is applied in the fields of medical science and chemical synthesis, can solve the problems of low bioavailability, poor solubility, and no obvious anti-tumor effect, and achieve good solubility and good bioavailability , significant antitumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
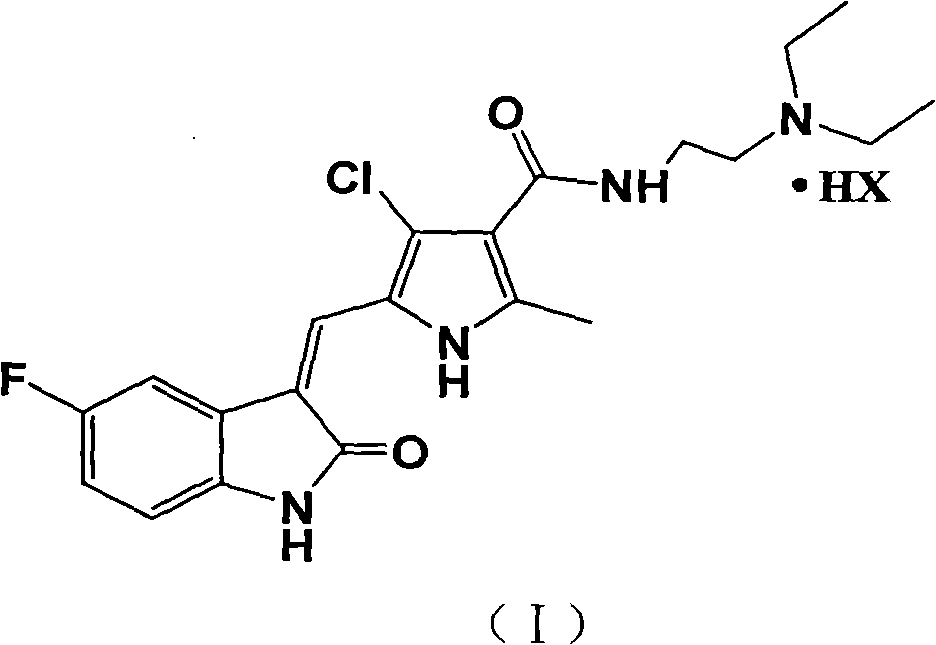
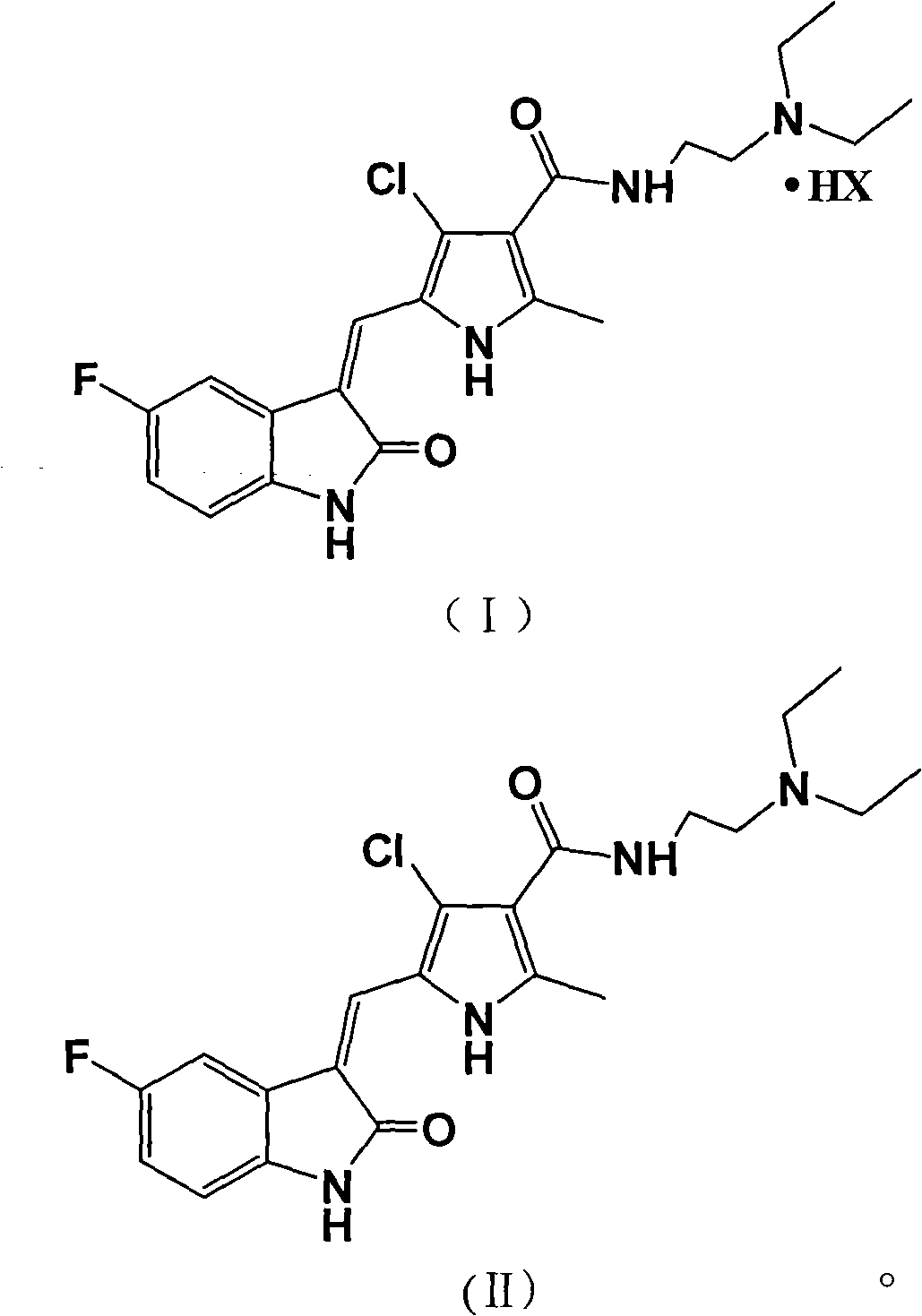
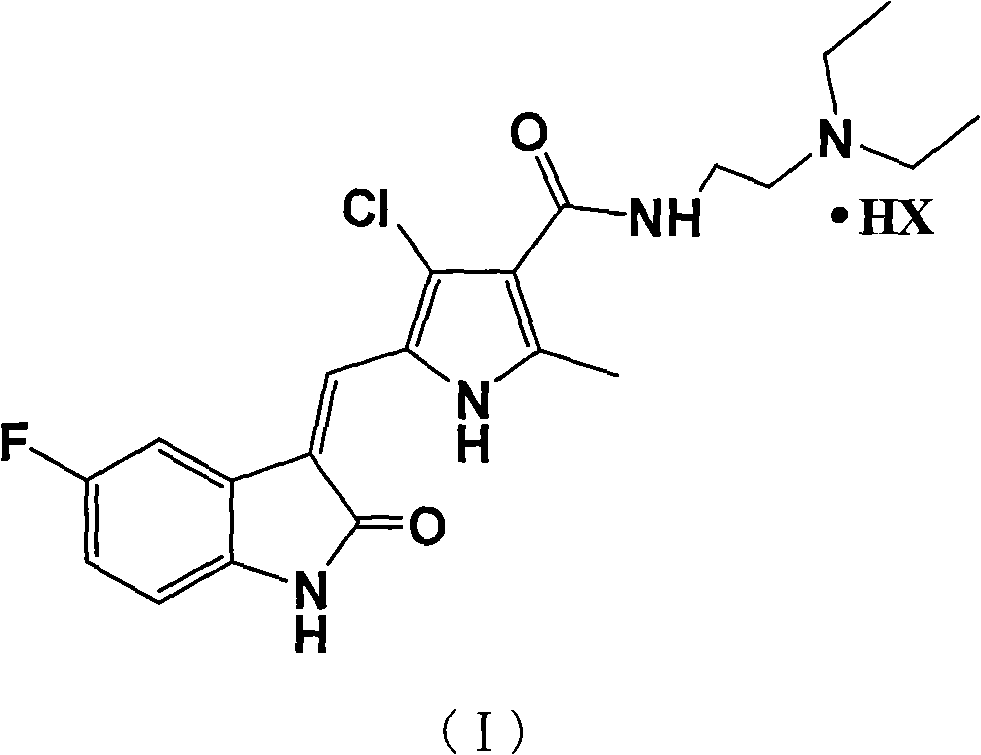
[0026] Example 1 N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indole-3-ylidene ) Methyl-2-methyl-4-chloro-1H-pyrrole-3-carboxamide hydrochloride
[0027] N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methanol Base-2-methyl-4-chloro-1H-pyrrole-3-carboxamide (1g, 2.4mmol) (formula II), absolute ethanol 10mL, concentrated hydrochloric acid 0.5mL were mixed and refluxed for 2h, and the precipitate was cooled and filtered. Dry in vacuum at 50°C for 2 hours to obtain 0.9 g of N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H- Indol-3-ylidene)methyl-2-methyl-4-chloro-1H-pyrrole-3-carboxamide hydrochloride. LC-MS (m / z): (M+1). mp: 248°C (decomposition).
[0028] 1 H NMR (D 2 O): 6.32-6.69(m, 5H), 3.25-3.53(m, 8H), 1.91(s, 3H) 1.33(t, 6H, J=7.2Hz).
[0029] Please refer to Chinese patent CN101440086A for the synthesis of formula (II) used in the present invention. Amine and 5-fluorooxindole condensation formed. ...
Embodiment 2
[0030] Example 2 N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indole-3-ylidene ) Methyl-2-methyl-4-chloro-1H-pyrrole-3-carboxamide L-tartrate
[0031] N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methanol Base-2-methyl-4-chloro-1H-pyrrole-3-carboxamide (1g, 2.4mmol) (formula II), absolute ethanol 10mL, L-tartaric acid (0.4g, 2.7mmol) were mixed and refluxed for 2h, cooled The precipitate was filtered, and the precipitate was vacuum-dried at 50° C. for 2 hours to obtain 0.95 g of N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fluoro-2-oxo-1,2 -dihydro-3H-indol-3-ylidene)methyl-2-methyl-4-chloro-1H-pyrrole-3-carboxamide L-tartrate. LC-MS (m / z): (M+1). mp: 162°C (decomposition).
[0032] 1 H NMR (D 2 O): 6.32-6.58(m, 5H), 4.43(s, 2H), 3.34-3.68(m, 6H), 2.65-2.98(m, 2H), 1.86(s, 3H), 1.34(t, 6H, J=7.2Hz).
Embodiment 3
[0033] Example 3 N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indole-3-ylidene ) Methyl-2-methyl-4-chloro-1H-pyrrole-3-carboxamide L-malate
[0034]N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methanol Base-2-methyl-4-chloro-1H-pyrrole-3-carboxamide (1g, 2.4mmol) (formula II), absolute ethanol 10mL, L-malic acid (0.38g, 2.8mmol) were mixed and refluxed for 2h, The precipitate was cooled and filtered, and the precipitate was vacuum-dried at 50°C for 2 hours to obtain 0.92 g of N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fluoro-2-oxo-1, 2-Dihydro-3H-indol-3-ylidene)methyl-2-methyl-4-chloro-1H-pyrrole-3-carboxamide L-malate. LC-MS (m / z): (M+1). mp: 203°C (decomposition).
[0035] 1 H NMR (D 2 O): 6.22-6.53(m, 5H), 4.40(s, 1H), 3.25-3.54(m, 8H), 2.62-2.86(m, 2H), 1.81(s, 3H), 1.30(t, 6H, J=7.2Hz).
[0036] One of the applied effects
[0037] 1. The halogenated pyrrole-substituted 2-indolinone malate prepared in Example 3 is su...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com